A 14-3-3 Protein-Encoding Gene, BdGF14g, Confers Better Drought Tolerance by Regulating ABA Biosynthesis and Signaling
- PMID: 38068611
- PMCID: PMC10707786
- DOI: 10.3390/plants12233975
A 14-3-3 Protein-Encoding Gene, BdGF14g, Confers Better Drought Tolerance by Regulating ABA Biosynthesis and Signaling
Abstract
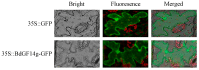
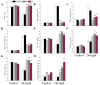
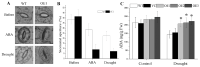
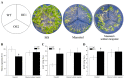
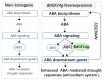
Abscisic acid (ABA), a phytohormone, enacts a cardinal function in coping with abiotic stress. 14-3-3 proteins can interact with ABA-responsive-element-binding transcription factors (ABFs), a chief constituent of ABA signaling, and play critical roles in the dehydration response involving ABA signaling. Meanwhile, whether and how 14-3-3 proteins regulate ABA signaling to respond to aridity stress is yet to be fully investigated. Herein, BdGF14g, a 14-3-3 gene induced by ABA, H2O2, and PEG treatments, was identified in Brachypodium distachyon (B. distachyon). Overexpression of BdGF14g improved drought stress tolerance in tobacco plants, with a higher survival rate, longer root length, enhanced cell membrane stability, and increased antioxidase activity compared with non-transgenic controls in coping with dehydration. Both drought and exogenous ABA treatments resulted in smaller stomatal apertures in BdGF14g-transgenic lines. Additionally, when an ABA biosynthesis inhibitor was added, the better growth statuses, less H2O2 accumulation, and higher activities of catalase and superoxide dismutase under mannitol stress disappeared. Moreover, BdGF14g interacted with NtABF2, upregulated the endogenous ABA content, and enhanced the transcription of ABA-related genes, including NtNCED1, a crucial ABA biosynthesis gene, under drought conditions. In conclusion, BdGF14g acts as a positive factor in the water deficiency response by affecting ABA biosynthesis and signaling in tobacco plants.
Keywords: BdGF14g; ROS-scavenging system; abscisic acid; drought stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
A Member of the 14-3-3 Gene Family in Brachypodium distachyon, BdGF14d, Confers Salt Tolerance in Transgenic Tobacco Plants.Front Plant Sci. 2017 Mar 13;8:340. doi: 10.3389/fpls.2017.00340. eCollection 2017. Front Plant Sci. 2017. PMID: 28348575 Free PMC article.
-
Sugarcane ScDREB2B-1 Confers Drought Stress Tolerance in Transgenic Nicotiana benthamiana by Regulating the ABA Signal, ROS Level and Stress-Related Gene Expression.Int J Mol Sci. 2022 Aug 23;23(17):9557. doi: 10.3390/ijms23179557. Int J Mol Sci. 2022. PMID: 36076957 Free PMC article.
-
The 14-3-3 Protein BdGF14a Increases the Transcriptional Regulation Activity of BdbZIP62 to Confer Drought and Salt Resistance in Tobacco.Plants (Basel). 2024 Jan 15;13(2):245. doi: 10.3390/plants13020245. Plants (Basel). 2024. PMID: 38256798 Free PMC article.
-
Cellular Phosphorylation Signaling and Gene Expression in Drought Stress Responses: ABA-Dependent and ABA-Independent Regulatory Systems.Plants (Basel). 2021 Apr 13;10(4):756. doi: 10.3390/plants10040756. Plants (Basel). 2021. PMID: 33924307 Free PMC article. Review.
-
Updates on the Role of ABSCISIC ACID INSENSITIVE 5 (ABI5) and ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTORs (ABFs) in ABA Signaling in Different Developmental Stages in Plants.Cells. 2021 Aug 5;10(8):1996. doi: 10.3390/cells10081996. Cells. 2021. PMID: 34440762 Free PMC article. Review.
Cited by
-
Construction of a Membrane Yeast Two-Hybrid Library and Screening of MsPYR1-Like Interacting Proteins in Malus sieversii.Mol Biotechnol. 2025 Jun;67(6):2319-2338. doi: 10.1007/s12033-024-01199-2. Epub 2024 Jun 2. Mol Biotechnol. 2025. PMID: 38824489
-
Polyethylene Glycol (PEG) Application Triggers Plant Dehydration but Does Not Accurately Simulate Drought.Plants (Basel). 2024 Dec 31;14(1):92. doi: 10.3390/plants14010092. Plants (Basel). 2024. PMID: 39795352 Free PMC article.
-
Development History, Structure, and Function of ASR (Abscisic Acid-Stress-Ripening) Transcription Factor.Int J Mol Sci. 2024 Sep 24;25(19):10283. doi: 10.3390/ijms251910283. Int J Mol Sci. 2024. PMID: 39408615 Free PMC article. Review.
References
-
- Jiang W., Tong T., Li W., Huang Z.H., Chen G., Zeng F.R., Riaz A., Amoanimaa-Dede H., Pan R., Zhang W.Y., et al. Molecular evolution of plant 14-3-3 proteins and function of Hv14-3-3A in stomatal regulation and drought tolerance. Plant Cell Physiol. 2023;63:1857–1872. doi: 10.1093/pcp/pcac034. - DOI - PubMed
LinkOut - more resources
Full Text Sources

