Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests
- PMID: 38068800
- PMCID: PMC10707750
- DOI: 10.3390/nu15234942
Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests
Abstract
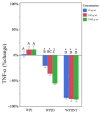
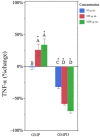
Whey protein isolate (WPI) consists of an array of proteins and peptides obtained as a byproduct of the cheesemaking process. Research suggests that WPI, along with its peptides such as glycomacropeptide (GMP), possesses immunomodulatory properties. These properties hold potential for alleviating the adverse effects of inflammatory conditions such as inflammatory bowel disease. Although promising, the immunoregulatory properties of the digested forms of WPI and GMP-those most likely to interact with the gut immune system-remain under-investigated. To address this knowledge gap, the current study examined the effects of in vitro-digested WPI and GMP, in vivo-digested WPI, and undigested WPI and GMP on the secretion of pro-inflammatory cytokines (TNF-α and IL-1β) in lipopolysaccharide-stimulated macrophage-like cells. Our results indicate that digested WPI and GMP reduced the expression of TNF-α and IL-1β, two pro-inflammatory cytokines. Whole WPI had no effect on TNF-α but reduced IL-1β levels. In contrast, in vivo-digested WPI reduced TNF-α but increased IL-1β. Undigested GMP, on the other hand, increased the secretion of both cytokines. These results demonstrate that digestion greatly modifies the effects of WPI and GMP on macrophages and suggest that digested WPI and GMP could help mitigate gastrointestinal inflammation. Further clinical studies are necessary to determine the biological relevance of WPI and GMP digestion products within the gut and their capacity to influence gut inflammation.
Keywords: Crohn’s disease; caseinmacropeptide; caseinomacropeptide; immunity; milk; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Postprandial metabolic responses to ingestion of bovine glycomacropeptide compared to a whey protein isolate in prediabetic volunteers.Eur J Nutr. 2019 Aug;58(5):2067-2077. doi: 10.1007/s00394-018-1763-5. Epub 2018 Jul 12. Eur J Nutr. 2019. PMID: 30003332
-
Commercial whey products promote intestinal barrier function with glycomacropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induced inflammation in vitro.Food Funct. 2020 Jul 22;11(7):5842-5852. doi: 10.1039/d0fo00487a. Food Funct. 2020. PMID: 32633745
-
Whey protein isolate and glycomacropeptide decrease weight gain and alter body composition in male Wistar rats.Br J Nutr. 2008 Jul;100(1):88-93. doi: 10.1017/S0007114507883000. Epub 2007 Dec 6. Br J Nutr. 2008. PMID: 18062832
-
Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns.Int J Biochem Cell Biol. 2013 Aug;45(8):1730-47. doi: 10.1016/j.biocel.2013.04.028. Epub 2013 May 6. Int J Biochem Cell Biol. 2013. PMID: 23660296 Review.
-
Potential Prebiotic Properties of Whey Protein and Glycomacropeptide in Gut Microbiome.Food Sci Anim Resour. 2024 Mar;44(2):299-308. doi: 10.5851/kosfa.2024.e12. Epub 2024 Mar 1. Food Sci Anim Resour. 2024. PMID: 38764509 Free PMC article. Review.
Cited by
-
Immunomodulatory Properties of Sweet Whey-Derived Peptides in THP-1 Macrophages.Molecules. 2025 Mar 11;30(6):1261. doi: 10.3390/molecules30061261. Molecules. 2025. PMID: 40142037 Free PMC article.
-
CSN1S1, CSN3 and LPL: Three Validated Gene Polymorphisms Useful for More Sustainable Dairy Production in the Mediterranean River Buffalo.Animals (Basel). 2024 May 9;14(10):1414. doi: 10.3390/ani14101414. Animals (Basel). 2024. PMID: 38791632 Free PMC article.
-
Casein Glycomacropeptide Regulates Gene Expression in Intestinal Epithelial Cells: Effect of Simulated Gastrointestinal Digestion and Peptide Microencapsulation.J Agric Food Chem. 2025 Feb 19;73(7):4105-4115. doi: 10.1021/acs.jafc.4c10146. Epub 2025 Feb 8. J Agric Food Chem. 2025. PMID: 39921639 Free PMC article.
-
Effect of Poly-γ-Glutamic Acid Molecular Weight on the Properties of Whey Protein Isolate Hydrogels.Polymers (Basel). 2025 Jun 9;17(12):1605. doi: 10.3390/polym17121605. Polymers (Basel). 2025. PMID: 40574133 Free PMC article.
References
-
- Madureira A.R., Pereira C.I., Gomes A.M.P., Pintado M.E., Xavier Malcata F. Bovine Whey Proteins—Overview on Their Main Biological Properties. Food Res. Int. 2007;40:1197–1211. doi: 10.1016/j.foodres.2007.07.005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

