Neuroprotective Effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-Induced Alzheimer's Disease Mouse Model
- PMID: 38068844
- PMCID: PMC10708322
- DOI: 10.3390/nu15234986
Neuroprotective Effects of N-methyl-(2S, 4R)-trans-4-hydroxy-L-proline (NMP) against Amyloid-β-Induced Alzheimer's Disease Mouse Model
Abstract
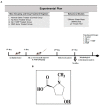
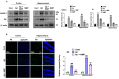
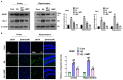
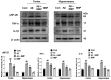
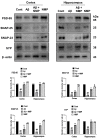
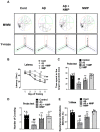
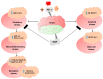
Alzheimer's disease (AD), is a progressive neurodegenerative disorder that involves the deposition of β-amyloid plaques and the clinical symptoms of confusion, memory loss, and cognitive dysfunction. Despite enormous progress in the field, no curative treatment is available. Therefore, the current study was designed to determine the neuroprotective effects of N-methyl-(2S, 4R)-Trans-4-hydroxy-L-proline (NMP) obtained from Sideroxylon obtusifolium, a Brazilian folk medicine with anti-inflammatory and anti-oxidative properties. Here, for the first time, we explored the neuroprotective role of NMP in the Aβ1-42-injected mouse model of AD. After acclimatization, a single intracerebroventricular injection of Aβ1-42 (5 µL/5 min/mouse) in C57BL/6N mice induced significant amyloidogenesis, reactive gliosis, oxidative stress, neuroinflammation, and synaptic and memory deficits. However, an intraperitoneal injection of NMP at a dose of (50 mg/kg/day) for three consecutive weeks remarkably decreased beta secretase1 (BACE-1) and Aβ, activated the astrocyte and microglia expression level as well as downstream inflammatory mediators such as pNF-ĸB, TNF-α, and IL-1β. NPM also strongly attenuated oxidative stress, as evaluated by the expression level of NRF2/HO-1, and synaptic failure, by improving the level of both the presynaptic (SNAP-25 and SYN) and postsynaptic (PSD-95 and SNAP-23) regions of the synapses in the cortexes and hippocampi of the Aβ1-42-injected mice, contributing to cognitive improvement in AD and improving the behavioral deficits displayed in the Morris water maze and Y-maze. Overall, our data suggest that NMP provides potent multifactorial effects, including the inhibition of amyloid plaques, oxidative stress, neuroinflammation, and cognitive deficits.
Keywords: Alzheimer’s disease; N-methyl-(2S, 4R)-Trans-4-hydroxy-L-proline (NMP); amyloid beta (Aβ1–42); neuroinflammation; neuroprotection; oxidative stress.
Conflict of interest statement
Author Myeong Ok Kim was employed by the company Alz-Dementia Korea Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Association A.S. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019;15:321–387. doi: 10.1016/j.jalz.2019.01.010. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

