Personalized Cancer Vaccines Go Viral: Viral Vectors in the Era of Personalized Immunotherapy of Cancer
- PMID: 38068911
- PMCID: PMC10706435
- DOI: 10.3390/ijms242316591
Personalized Cancer Vaccines Go Viral: Viral Vectors in the Era of Personalized Immunotherapy of Cancer
Abstract
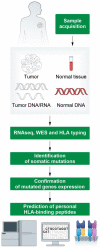
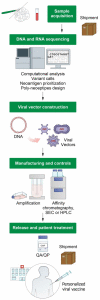
The aim of personalized cancer vaccines is to elicit potent and tumor-specific immune responses against neoantigens specific to each patient and to establish durable immunity, while minimizing the adverse events. Over recent years, there has been a renewed interest in personalized cancer vaccines, primarily due to the advancement of innovative technologies for the identification of neoantigens and novel vaccine delivery platforms. Here, we review the emerging field of personalized cancer vaccination, with a focus on the use of viral vectors as a vaccine platform. The recent advancements in viral vector technology have led to the development of efficient production processes, positioning personalized viral vaccines as one of the preferred technologies. Many clinical trials have shown the feasibility, safety, immunogenicity and, more recently, preliminary evidence of the anti-tumor activity of personalized vaccination, fostering active research in the field, including further clinical trials for different tumor types and in different clinical settings.
Keywords: adenovirus; neoantigens; personalized cancer vaccines; poxvirus; viral vectors.
Conflict of interest statement
E.S. is the founder of Nouscom. L.S., G.L., V.R., L.S., G.C., E.S. and A.M.D. are employed by Nouscom.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

