Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro
- PMID: 38069038
- PMCID: PMC10706138
- DOI: 10.3390/ijms242316713
Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro
Abstract
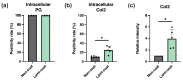
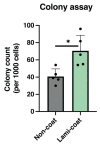
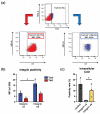
The angiopoietin-1 receptor (Tie2) marks specific nucleus pulposus (NP) progenitor cells, shows a rapid decline during aging and intervertebral disc degeneration, and has thus sparked interest in its utilization as a regenerative agent against disc degeneration. However, the challenge of maintaining and expanding these progenitor cells in vitro has been a significant hurdle. In this study, we investigated the potential of laminin-511 to sustain Tie2+ NP progenitor cells in vitro. We isolated cells from human NP tissue (n = 5) and cultured them for 6 days on either standard (Non-coat) or iMatrix-511 (laminin-511 product)-coated (Lami-coat) dishes. We assessed these cells for their proliferative capacity, activation of Erk1/2 and Akt pathways, as well as the expression of cell surface markers such as Tie2, GD2, and CD24. To gauge their regenerative potential, we examined their extracellular matrix (ECM) production capacity (intracellular type II collagen (Col2) and proteoglycans (PG)) and their ability to form spherical colonies within methylcellulose hydrogels. Lami-coat significantly enhanced cell proliferation rates and increased Tie2 expression, resulting in a 7.9-fold increase in Tie2-expressing cell yields. Moreover, the overall proportion of cells positive for Tie2 also increased 2.7-fold. Notably, the Col2 positivity rate was significantly higher on laminin-coated plates (Non-coat: 10.24% (±1.7%) versus Lami-coat: 26.2% (±7.5%), p = 0.010), and the ability to form spherical colonies also showed a significant improvement (Non-coat: 40.7 (±8.8)/1000 cells versus Lami-coat: 70.53 (±18.0)/1000 cells, p = 0.016). These findings demonstrate that Lami-coat enhances the potential of NP cells, as indicated by improved colony formation and proliferative characteristics. This highlights the potential of laminin-coating in maintaining the NP progenitor cell phenotype in culture, thereby supporting their translation into prospective clinical cell-transplantation products.
Keywords: Tie2; cell proliferation; iMatrix-511; intervertebral disc; laminin-511; nucleus pulposus; progenitor cell; regenerative potential; type 2 collagen.
Conflict of interest statement
Author D.S. is a scientific advisor for TUNZ Pharma Co., Ltd. (Osaka, Japan). Authors H.S., T.W., and M.N. are employed members of TUNZ Pharma Co., Ltd. (Osaka, Japan). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
-
- Fainor M., Orozco B.S., Muir V.G., Mahindroo S., Gupta S., Mauck R.L., Burdick J.A., Smith H.E., Gullbrand S.E. Mechanical crosstalk between the intervertebral disc, facet joints, and vertebral endplate following acute disc injury in a rabbit model. JOR Spine. 2023:e1287. doi: 10.1002/jsp2.1287. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

