Allergenic Activity of Individual Cat Allergen Molecules
- PMID: 38069052
- PMCID: PMC10706119
- DOI: 10.3390/ijms242316729
Allergenic Activity of Individual Cat Allergen Molecules
Abstract
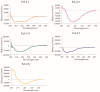
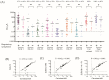
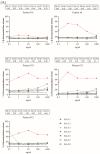
More than 10% of the world's population suffers from an immunoglobulin E (IgE)-mediated allergy to cats which is accompanied mainly by respiratory symptoms such as rhinitis and asthma. Several cat allergen molecules have been identified, but their allergenic activity has not been investigated in depth. Purified cat allergen molecules (Fel d 1, Fel d 2, Fel d 3, Fel d 4, Fel d 6, Fel d 7 and Fel d 8) were characterized via mass spectrometry and circular dichroism spectroscopy regarding their molecular mass and fold, respectively. Cat-allergen-specific IgE levels were quantified via ImmunoCAP measurements in IgE-sensitized subjects with (n = 37) and without (n = 20) respiratory symptoms related to cat exposure. The allergenic activity of the cat allergens was investigated by loading patients' IgE onto rat basophils expressing the human FcεRI receptor and studying the ability of different allergen concentrations to induce β-hexosaminidase release. Purified and folded cat allergens with correct masses were obtained. Cat-allergen-specific IgE levels were much higher in patients with a respiratory allergy than in patients without a respiratory allergy. Fel d 1, Fel d 2, Fel d 4 and Fel d 7 bound the highest levels of specific IgE and already-induced basophil degranulation at hundred-fold-lower concentrations than the other allergens. Fel d 1, Fel d 4 and Fel d 7 were recognized by more than 65% of patients with a respiratory allergy, whereas Fel d 2 was recognized by only 30%. Therefore, in addition to the major cat allergen Fel d 1, Fel d 4 and Fel d 7 should also be considered to be important allergens for the diagnosis and specific immunotherapy of cat allergy.
Keywords: IgE reactivity; allergenic activity; allergy; basophil activation test; cat allergen molecule; cat allergy.
Conflict of interest statement
R.V. received research grants from HiPP GmbH & Co., Vertrieb KG, Pfaffenhofen, Germany; Worg Pharmaceuticals, Hangzhou, China; HVD Biotech, Vienna, Austria; and Viravaxx, Vienna, Austria. He serves as a consultant for Viravaxx and Worg. D.T., M.C., K.R., A.K. (Antonina Karsonova), M.v.H., A.K. (Alexander Karaulov) and R.V. are authors of the patent application “Vaccine for treating allergies” application number: PCT/CN2022/130414, reference: P20222370. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; or in the decision to publish the results. The authors with Russian affiliation declare that they have prepared the article in their “personal capacity” and/or that they are employed at an academic/research institution where research or education is the primary function of the entity.
Figures

References
-
- Heinzerling L.M., Burbach G.J., Edenharter G., Bachert C., Bindslev-Jensen C., Bonini S., Bousquet J., Bousquet-Rouanet L., Bousquet P.J., Bresciani M., et al. GA2LEN skin test study I: GA2LEN harmonization of skin prick testing: Novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–1506. doi: 10.1111/j.1398-9995.2009.02093.x. - DOI - PubMed
-
- D’souza N., Weber M., Sarzsinszky E., Vrtala S., Curin M., Schaar M., Garib V., Focke-Tejkl M., Li Y., Jones R., et al. The Molecular Allergen Recognition Profile in China as Basis for Allergen-Specific Immunotherapy. Front. Immunol. 2021;12:719573. doi: 10.3389/fimmu.2021.719573. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

