miR-877-5p as a Potential Link between Triple-Negative Breast Cancer Development and Metabolic Syndrome
- PMID: 38069080
- PMCID: PMC10706566
- DOI: 10.3390/ijms242316758
miR-877-5p as a Potential Link between Triple-Negative Breast Cancer Development and Metabolic Syndrome
Abstract
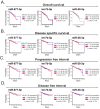
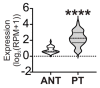
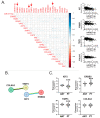
Metabolic syndrome (MS) is a risk factor for breast cancer (BC) that increases its aggressiveness and metastasis. The prevalence of MS is higher in triple-negative breast cancer (TNBC), which is the molecular subtype with the worst prognosis. The molecular mechanisms underlying this association have not been fully elucidated. MiRNAs are small, non-coding RNAs that regulate gene expression. Aberrant expression of miRNAs in both tissues and fluids are linked to several pathologies. The aim of this work was to identify circulating miRNAs in patients with alterations associated with MS (AAMS) that also impact on BC. Using microarray technology, we detected 23 miRNAs altered in the plasma of women with AAMS that modulate processes linked to cancer. We found that let-7b-5p and miR-28-3p were decreased in plasma from patients with AAMS and also in BC tumors, while miR-877-5p was increased. Interestingly, miR-877-5p expression was associated with lower patient survival, and its expression was higher in PAM50 basal-like BC tumors compared to the other molecular subtypes. Analyses from public databases revealed that miR-877-5p was also increased in plasma from BC patients compared to plasma from healthy donors. We identified IGF2 and TIMP3 as validated target genes of miR-877-5p whose expression was decreased in BC tissue and moreover, was negatively correlated with the levels of this miRNA in the tumors. Finally, a miRNA inhibitor against miR-877-5p diminished viability and tumor growth of the TNBC model 4T1. These results reveal that miR-877-5p inhibition could be a therapeutic option for the treatment of TNBC. Further studies are needed to investigate the role of this miRNA in TNBC progression.
Keywords: breast cancer; metabolic syndrome; miRNAs.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

