Cracking the Endothelial Calcium (Ca2+) Code: A Matter of Timing and Spacing
- PMID: 38069089
- PMCID: PMC10706333
- DOI: 10.3390/ijms242316765
Cracking the Endothelial Calcium (Ca2+) Code: A Matter of Timing and Spacing
Abstract
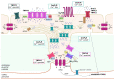
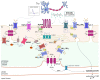
A monolayer of endothelial cells lines the innermost surface of all blood vessels, thereby coming into close contact with every region of the body and perceiving signals deriving from both the bloodstream and parenchymal tissues. An increase in intracellular Ca2+ concentration ([Ca2+]i) is the main mechanism whereby vascular endothelial cells integrate the information conveyed by local and circulating cues. Herein, we describe the dynamics and spatial distribution of endothelial Ca2+ signals to understand how an array of spatially restricted (at both the subcellular and cellular levels) Ca2+ signals is exploited by the vascular intima to fulfill this complex task. We then illustrate how local endothelial Ca2+ signals affect the most appropriate vascular function and are integrated to transmit this information to more distant sites to maintain cardiovascular homeostasis. Vasorelaxation and sprouting angiogenesis were selected as an example of functions that are finely tuned by the variable spatio-temporal profile endothelial Ca2+ signals. We further highlighted how distinct Ca2+ signatures regulate the different phases of vasculogenesis, i.e., proliferation and migration, in circulating endothelial precursors.
Keywords: Ca2+ oscillations; Ca2+ pulsars; Ca2+ signaling; Ca2+ sparklets; Ca2+ wavelets; Ca2+ waves; angiogenesis; endothelial cells; myo-endothelial gap junctions; vasorelaxation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- MNESYS (PE0000006) - A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022)/Ministry of University and Research
- LION-HEARTED (Grant Agreement N. 828984)/EU Horizon 2020 FETOPEN-2018-2020 Program
- Fondo Ricerca Giovani/University of Pavia
- CVU121216/CONACYT
LinkOut - more resources
Full Text Sources
Miscellaneous

