Molecular Mechanisms of the Regulation of Liver Cytochrome P450 by Brain NMDA Receptors and via the Neuroendocrine Pathway-A Significance for New Psychotropic Therapies
- PMID: 38069162
- PMCID: PMC10706700
- DOI: 10.3390/ijms242316840
Molecular Mechanisms of the Regulation of Liver Cytochrome P450 by Brain NMDA Receptors and via the Neuroendocrine Pathway-A Significance for New Psychotropic Therapies
Abstract
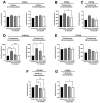
Recent investigations have highlighted the potential utility of the selective antagonist of the NMDA receptor GluN2B subunit for addressing major depressive disorders. Our previous study showed that the systemic administration of the antagonist of the GluN2B subunit of the NMDA receptor, the compound CP-101,606, affected liver cytochrome P450 expression and activity. To discern between the central and peripheral mechanisms of enzyme regulation, our current study aimed to explore whether the intracerebral administration of CP-101,606 could impact cytochrome P450. The injection of CP-101,606 to brain lateral ventricles (6, 15, or 30 µg/brain) exerted dose-dependent effects on liver cytochrome P450 enzymes and hypothalamic or pituitary hormones. The lowest dose led to an increase in the activity, protein, and mRNA level of CYP2C11 compared to the control. The activities of CYP2A, CYP2B, CYP2C11, CYP2C6, CYP2D, and protein levels of CYP2B, CYP2C11 were enhanced compared to the highest dose. Moreover, CP-101,606 increased the CYP1A protein level coupled with elevated CYP1A1 and CYP1A2 mRNA levels, but not activity. The antagonist decreased the pituitary somatostatin level and increased the serum growth hormone concentration after the lowest dose, while independently decreasing the serum corticosterone concentration of the dose. The findings presented here unveil a novel physiological regulatory mechanism whereby the brain glutamatergic system, via the NMDA receptor, influences liver cytochrome P450. This regulatory process appears to involve the endocrine system. These results may have practical applications in predicting alterations in cytochrome P450 activity and endogenous metabolism, and potential metabolic drug-drug interactions elicited by drugs that cross the blood-brain barrier and affect NMDA receptors.
Keywords: CP-101,606; NMDA receptor; activity/expression; cytochrome P450; liver; neuroendocrine regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The neuroendocrine regulation of hepatic cytochrome P450 by N-methyl-D-aspartate receptors in the paraventricular and arcuate nuclei of the hypothalamus.Drug Metab Dispos. 2025 Aug 6;53(9):100143. doi: 10.1016/j.dmd.2025.100143. Online ahead of print. Drug Metab Dispos. 2025. PMID: 40882442
-
The Selective NMDA Receptor GluN2B Subunit Antagonist CP-101,606 with Antidepressant Properties Modulates Cytochrome P450 Expression in the Liver.Pharmaceutics. 2021 Oct 9;13(10):1643. doi: 10.3390/pharmaceutics13101643. Pharmaceutics. 2021. PMID: 34683936 Free PMC article.
-
The Effect of the Selective N-methyl-D-aspartate (NMDA) Receptor GluN2B Subunit Antagonist CP-101,606 on Cytochrome P450 2D (CYP2D) Expression and Activity in the Rat Liver and Brain.Int J Mol Sci. 2022 Nov 8;23(22):13746. doi: 10.3390/ijms232213746. Int J Mol Sci. 2022. PMID: 36430225 Free PMC article.
-
The mechanisms of interactions of psychotropic drugs with liver and brain cytochrome P450 and their significance for drug effect and drug-drug interactions.Biochem Pharmacol. 2022 May;199:115006. doi: 10.1016/j.bcp.2022.115006. Epub 2022 Mar 18. Biochem Pharmacol. 2022. PMID: 35314167 Review.
-
The role of the nervous system in the regulation of liver cytochrome p450.Curr Drug Metab. 2011 Feb;12(2):124-38. doi: 10.2174/138920011795016908. Curr Drug Metab. 2011. PMID: 21401511 Review.
Cited by
-
Cannabinoids and the endocannabinoid system in the regulation of cytochrome P450 metabolic activity-a review.Front Pharmacol. 2025 Jun 5;16:1599012. doi: 10.3389/fphar.2025.1599012. eCollection 2025. Front Pharmacol. 2025. PMID: 40538541 Free PMC article. Review.
References
-
- Hines R.N., Luo Z., Cresteil T., Ding X., Prough R.A., Fitzpatrick J.L., Ripp S.L., Falkner K.C., Ge N.-L., Levine A., et al. Molecular Regulation of Genes Encoding Xenobiotic-Metabolizing Enzymes: Mechanisms Involving Endogenous Factors. Drug Metab. Dispos. 2001;29:623–633. - PubMed
-
- Pascussi J.-M., Gerbal-Chaloin S., Duret C., Daujat-Chavanieu M., Vilarem M.-J., Maurel P. The Tangle of Nuclear Receptors That Controls Xenobiotic Metabolism and Transport: Crosstalk and Consequences. Annu. Rev. Pharmacol. Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

