Comparative Analysis of Morphological and Functional Effects of 225Ac- and 177Lu-PSMA Radioligand Therapies (RLTs) on Salivary Glands
- PMID: 38069166
- PMCID: PMC10706561
- DOI: 10.3390/ijms242316845
Comparative Analysis of Morphological and Functional Effects of 225Ac- and 177Lu-PSMA Radioligand Therapies (RLTs) on Salivary Glands
Abstract
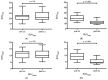
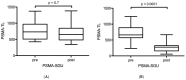
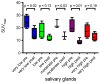
Most Prostate Specific Membrane Antigens (PSMAs) targeting small molecules accumulate in the salivary glands (SGs), raising concerns about SG toxicity, especially after repeated therapies or therapy with 225Ac-labeled ligands. SG toxicity is assessed clinically by the severity of patient-reported xerostomia, but this parameter can be challenging to objectively quantify. Therefore, we explored the feasibility of using SG volume as a biomarker for toxicity. In 21 patients with late-stage metastatic resistant prostate cancer (mCRPC), the PSMA volume and ligand uptake of SG were analyzed retrospectively before and after two cycles of 177Lu-PSMA (LuPSMA; cohort A) and before and after one cycle of 225Ac-PSMA-617 (AcPSMA, cohort B). Mean Volume-SG in cohort A was 59 ± 13 vs. 54 ± 16 mL (-10%, p = 0.4), and in cohort B, it was 50 ± 13 vs. 40 ± 11 mL (-20%, p = 0.007), respectively. A statistically significant decrease in the activity concentration in the SG was only observed in group B (SUVmean: 9.2 ± 2.8 vs. 5.3 ± 1.8, p < 0.0001; vs. A: SUVmean: 11.2 ± 3.3 vs. 11.1 ± 3.5, p = 0.8). SG volume and PSMA-ligand uptake are promising markers to monitor the SG toxicity after a PSMA RLT.
Keywords: Actinium-225-PSMA-617; PSMA; mCRPC; radioligand therapy; salivary glands; tumor sink effect; xerostomia.
Conflict of interest statement
ME reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker), Janssen Pharmaceuticals (consultant, speakers bureau), Parexel (image review), and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. BF reports fees from Novartis (consultant). WW reports that he is on advisory boards and receives compensation from Bayer, Blue Earth Diagnostics, Endocyte, Reflexion, Rayzebio, Vida Ventures, ITM, and Pentixapharm. He has received research support from Siemens, BMS, Ipsen, Imaginab, and Piramal. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Figures

References
-
- Rahbar K., Ahmadzadehfar H., Kratochwil C., Haberkorn U., Schafers M., Essler M., Baum R.P., Kulkarni H.R., Schmidt M., Drzezga A., et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. - DOI - PubMed
-
- Kratochwil C., Bruchertseifer F., Rathke H., Hohenfellner M., Giesel F.L., Haberkorn U., Morgenstern A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018;59:795–802. doi: 10.2967/jnumed.117.203539. - DOI - PubMed
-
- Kratochwil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. - DOI - PubMed
-
- Zacherl M.J., Gildehaus F.J., Mittlmeier L., Boning G., Gosewisch A., Wenter V., Unterrainer M., Schmidt-Hegemann N., Belka C., Kretschmer A., et al. First Clinical Results for PSMA-Targeted alpha-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021;62:669–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

