Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise
- PMID: 38069198
- PMCID: PMC10706777
- DOI: 10.3390/ijms242316870
Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise
Abstract
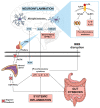
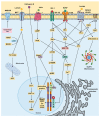
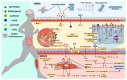
Major depressive disorder (MDD) has a high prevalence and is a major contributor to the global burden of disease. This psychiatric disorder results from a complex interaction between environmental and genetic factors. In recent years, the role of the gut microbiota in brain health has received particular attention, and compelling evidence has shown that patients suffering from depression have gut dysbiosis. Several studies have reported that gut dysbiosis-induced inflammation may cause and/or contribute to the development of depression through dysregulation of the gut-brain axis. Indeed, as a consequence of gut dysbiosis, neuroinflammatory alterations caused by microglial activation together with impairments in neuroplasticity may contribute to the development of depressive symptoms. The modulation of the gut microbiota has been recognized as a potential therapeutic strategy for the management of MMD. In this regard, physical exercise has been shown to positively change microbiota composition and diversity, and this can underlie, at least in part, its antidepressant effects. Given this, the present review will explore the relationship between physical exercise, gut microbiota and depression, with an emphasis on the potential of physical exercise as a non-invasive strategy for modulating the gut microbiota and, through this, regulating the gut-brain axis and alleviating MDD-related symptoms.
Keywords: gut microbiota; major depressive disorder; physical exercise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Campbell D., Green M.J., Davies N., Demou E., Howe L.D., Harrison S., Smith D.J., Howard D.M., McIntosh A.M., Munafò M., et al. Effects of depression on employment and social outcomes: A Mendelian randomisation study. J. Epidemiol. Community Health. 2022;76:563–571. doi: 10.1136/jech-2021-218074. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

