Cellular Responses to Widespread DNA Replication Stress
- PMID: 38069223
- PMCID: PMC10707325
- DOI: 10.3390/ijms242316903
Cellular Responses to Widespread DNA Replication Stress
Abstract
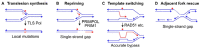
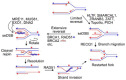
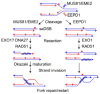
Replicative DNA polymerases are blocked by nearly all types of DNA damage. The resulting DNA replication stress threatens genome stability. DNA replication stress is also caused by depletion of nucleotide pools, DNA polymerase inhibitors, and DNA sequences or structures that are difficult to replicate. Replication stress triggers complex cellular responses that include cell cycle arrest, replication fork collapse to one-ended DNA double-strand breaks, induction of DNA repair, and programmed cell death after excessive damage. Replication stress caused by specific structures (e.g., G-rich sequences that form G-quadruplexes) is localized but occurs during the S phase of every cell division. This review focuses on cellular responses to widespread stress such as that caused by random DNA damage, DNA polymerase inhibition/nucleotide pool depletion, and R-loops. Another form of global replication stress is seen in cancer cells and is termed oncogenic stress, reflecting dysregulated replication origin firing and/or replication fork progression. Replication stress responses are often dysregulated in cancer cells, and this too contributes to ongoing genome instability that can drive cancer progression. Nucleases play critical roles in replication stress responses, including MUS81, EEPD1, Metnase, CtIP, MRE11, EXO1, DNA2-BLM, SLX1-SLX4, XPF-ERCC1-SLX4, Artemis, XPG, FEN1, and TATDN2. Several of these nucleases cleave branched DNA structures at stressed replication forks to promote repair and restart of these forks. We recently defined roles for EEPD1 in restarting stressed replication forks after oxidative DNA damage, and for TATDN2 in mitigating replication stress caused by R-loop accumulation in BRCA1-defective cells. We also discuss how insights into biological responses to genome-wide replication stress can inform novel cancer treatment strategies that exploit synthetic lethal relationships among replication stress response factors.
Keywords: DNA damage; DNA damage response; DNA double-strand breaks; genome instability; oxidative DNA damage; replication stress; structure-specific nucleases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

