Multi-Organ Increase in Norepinephrine Levels after Central Leptin Administration and Diet-Induced Obesity
- PMID: 38069231
- PMCID: PMC10706686
- DOI: 10.3390/ijms242316909
Multi-Organ Increase in Norepinephrine Levels after Central Leptin Administration and Diet-Induced Obesity
Abstract
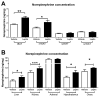
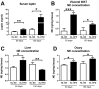
Autonomic innervation is important to regulate homeostasis in every organ of the body. The sympathetic nervous system controls several organs associated with metabolism and reproduction, including adipose tissue, the liver, and the ovaries. The sympathetic nervous system is controlled within the central nervous system by neurons located in the hypothalamus, which in turn are regulated by hormones like leptin. Leptin action in the hypothalamus leads to increased sympathetic activity in the adipose tissue. In this short report, we propose that leptin action in the brain also controls the sympathetic innervation of other organs like the liver and the ovary. We performed two experiments: We performed an intracerebroventricular (ICV) injection of leptin and measured norepinephrine levels in several organs, and we used a validated model of overnutrition and obesity to evaluate whether an increase in leptin levels coexists with high levels of norepinephrine in the liver and ovaries. Norepinephrine was measured by ELISA in adipose tissue and by HPLC-EC in other tissues. Leptin was measured by ELISA. We found that the ICV injection of leptin increases norepinephrine levels in several organs, including the liver and ovaries. Also, we found that diet-induced obesity leads to an increase in leptin levels while inducing an increase in norepinephrine levels in the liver and ovaries. Finally, since hyperactivity of the sympathetic nervous system is observed both in non-alcoholic fatty liver disease and polycystic ovary syndrome, we think that an increase in norepinephrine levels induced by hyperleptinemia could be involved in the pathogenesis of both diseases.
Keywords: fat; leptin; liver; norepinephrine; ovary; sympathetic nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

