The Haptoglobin Response after Aneurysmal Subarachnoid Haemorrhage
- PMID: 38069244
- PMCID: PMC10707007
- DOI: 10.3390/ijms242316922
The Haptoglobin Response after Aneurysmal Subarachnoid Haemorrhage
Abstract
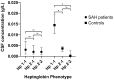
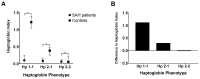
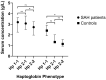
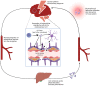
Haptoglobin is the body's first line of defence against the toxicity of extracellular haemoglobin released following a subarachnoid haemorrhage (SAH). We investigated the haptoglobin response after SAH in cerebrospinal fluid (CSF) and serum. Paired CSF and serum samples from 19 controls and 92 SAH patients were assayed as follows: ultra-performance liquid chromatography for CSF haemoglobin and haptoglobin, immunoassay for serum haptoglobin and multiplexed CSF cytokines, and colorimetry for albumin. There was marked CSF haptoglobin deficiency: 99% of extracellular haemoglobin was unbound. The quotients for both CSF/serum albumin (qAlb) and haptoglobin (qHp) were used to compute the CSF haptoglobin index (qHp/qAlb). CSF from SAH patients had a significantly lower haptoglobin index compared to controls, especially in Haptoglobin-1 allele carriers. Serum haptoglobin levels increased after SAH and were correlated with CSF cytokine levels. Haptoglobin variables were not associated with long-term clinical outcomes post-SAH. We conclude that: (1) intrathecal haptoglobin consumption occurs after SAH, more so in haptoglobin-1 allele carriers; (2) serum haptoglobin is upregulated after SAH, in keeping with the liver acute phase response to central inflammation; (3) haptoglobin in the CSF is so low that any variation is too small for this to affect long-term outcomes, emphasising the potential for therapeutic haptoglobin supplementation.
Keywords: cerebrospinal fluid; cytokines; haemoglobin; haptoglobin; subarachnoid haemorrhage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

