Socrates: A Novel N-Ethyl-N-nitrosourea-Induced Mouse Mutant with Audiogenic Epilepsy
- PMID: 38069426
- PMCID: PMC10707124
- DOI: 10.3390/ijms242317104
Socrates: A Novel N-Ethyl-N-nitrosourea-Induced Mouse Mutant with Audiogenic Epilepsy
Abstract
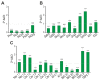
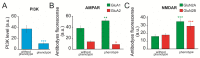
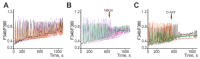
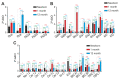
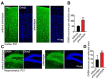
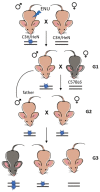
Epilepsy is one of the common neurological diseases that affects not only adults but also infants and children. Because epilepsy has been studied for a long time, there are several pharmacologically effective anticonvulsants, which, however, are not suitable as therapy for all patients. The genesis of epilepsy has been extensively investigated in terms of its occurrence after injury and as a concomitant disease with various brain diseases, such as tumors, ischemic events, etc. However, in the last decades, there are multiple reports that both genetic and epigenetic factors play an important role in epileptogenesis. Therefore, there is a need for further identification of genes and loci that can be associated with higher susceptibility to epileptic seizures. Use of mouse knockout models of epileptogenesis is very informative, but it has its limitations. One of them is due to the fact that complete deletion of a gene is not, in many cases, similar to human epilepsy-associated syndromes. Another approach to generating mouse models of epilepsy is N-Ethyl-N-nitrosourea (ENU)-directed mutagenesis. Recently, using this approach, we generated a novel mouse strain, soc (socrates, formerly s8-3), with epileptiform activity. Using molecular biology methods, calcium neuroimaging, and immunocytochemistry, we were able to characterize the strain. Neurons isolated from soc mutant brains retain the ability to differentiate in vitro and form a network. However, soc mutant neurons are characterized by increased spontaneous excitation activity. They also demonstrate a high degree of Ca2+ activity compared to WT neurons. Additionally, they show increased expression of NMDA receptors, decreased expression of the Ca2+-conducting GluA2 subunit of AMPA receptors, suppressed expression of phosphoinositol 3-kinase, and BK channels of the cytoplasmic membrane involved in protection against epileptogenesis. During embryonic and postnatal development, the expression of several genes encoding ion channels is downregulated in vivo, as well. Our data indicate that soc mutation causes a disruption of the excitation-inhibition balance in the brain, and it can serve as a mouse model of epilepsy.
Keywords: calcium ions; epileptiform activity; gene expression; mutagenesis; neurons; receptors; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Symonds J.D., Zuberi S.M., Stewart K., McLellan A., O’Regan M., MacLeod S., Jollands A., Joss S., Kirkpatrick M., Brunklaus A., et al. Incidence and Phenotypes of Childhood-Onset Genetic Epilepsies: A Prospective Population-Based National Cohort. Brain. 2019;142:2303–2318. doi: 10.1093/brain/awz195. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

