FXTAS Neuropathology Includes Widespread Reactive Astrogliosis and White Matter Specific Astrocyte Degeneration
- PMID: 38069470
- PMCID: PMC10922917
- DOI: 10.1002/ana.26851
FXTAS Neuropathology Includes Widespread Reactive Astrogliosis and White Matter Specific Astrocyte Degeneration
Abstract
Objective: Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset progressive genetic neurodegenerative disorder that occurs in FMR1 premutation carriers. The temporal, spatial, and cell-type specific patterns of neurodegeneration in the FXTAS brain remain incompletely characterized. Intranuclear inclusion bodies are the neuropathological hallmark of FXTAS, which are largest and occur most frequently in astrocytes, glial cells that maintain brain homeostasis. Here, we characterized neuropathological alterations in astrocytes in multiple regions of the FXTAS brain.
Methods: Striatal and cerebellar sections from FXTAS cases (n = 12) and controls (n = 12) were stained for the astrocyte markers glial fibrillary acidic protein (GFAP) and aldehyde dehydrogenase 1L1 (ALDH1L1) using immunohistochemistry. Reactive astrogliosis severity, the prevalence of GFAP+ fragments, and astrocyte density were scored. Double label immunofluorescence was utilized to detect co-localization of GFAP and cleaved caspase-3.
Results: FXTAS cases showed widespread reactive gliosis in both grey and white matter. GFAP staining also revealed remarkably severe astrocyte pathology in FXTAS white matter - characterized by a significant and visible reduction in astrocyte density (-38.7% in striatum and - 32.2% in cerebellum) and the widespread presence of GFAP+ fragments reminiscent of apoptotic bodies. White matter specific reductions in astrocyte density were confirmed with ALDH1L1 staining. GFAP+ astrocytes and fragments in white matter were positive for cleaved caspase-3, suggesting that apoptosis-mediated degeneration is responsible for reduced astrocyte counts.
Interpretation: We have established that FXTAS neuropathology includes robust degeneration of astrocytes, which is specific to white matter. Because astrocytes are essential for maintaining homeostasis within the central nervous system, a loss of astrocytes likely further exacerbates neuropathological progression of other cell types in the FXTAS brain. ANN NEUROL 2024;95:558-575.
© 2023 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest
BDD, TB, EM, EA, YM, and VMC do not have any real or apparent conflicts of interest to disclose.
Figures





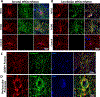
Comment in
-
White matter astrocyte degeneration in FXTAS.Nat Rev Neurol. 2024 Feb;20(2):63. doi: 10.1038/s41582-023-00924-w. Nat Rev Neurol. 2024. PMID: 38167678 No abstract available.
References
-
- Hagerman PJ, Greco CM, Hagerman RJ. A cerebellar tremor/ataxia syndrome among fragile X premutation carriers. Cytogenet Genome Res. 2003;100(1–4):206–12. - PubMed
-
- Hagerman RJ, Hagerman P. Fragile X-associated tremor/ataxia syndrome - features, mechanisms and management. Nat Rev Neurol. 2016. Jul;12(7):403–12. - PubMed
-
- Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008. Jan;22(1):48–60. - PubMed
-
- Hagerman RJ, Coffey SM, Maselli R, et al. Neuropathy as a presenting feature in fragile X-associated tremor/ataxia syndrome. Am J Med Genet A. 2007. Oct 1;143A(19):2256–60. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

