A tridimensional atlas of the developing human head
- PMID: 38070509
- PMCID: PMC10783631
- DOI: 10.1016/j.cell.2023.11.013
A tridimensional atlas of the developing human head
Abstract
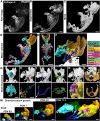
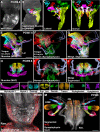
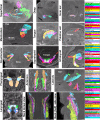
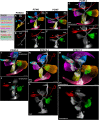
The evolution and development of the head have long captivated researchers due to the crucial role of the head as the gateway for sensory stimuli and the intricate structural complexity of the head. Although significant progress has been made in understanding head development in various vertebrate species, our knowledge of early human head ontogeny remains limited. Here, we used advanced whole-mount immunostaining and 3D imaging techniques to generate a comprehensive 3D cellular atlas of human head embryogenesis. We present detailed developmental series of diverse head tissues and cell types, including muscles, vasculature, cartilage, peripheral nerves, and exocrine glands. These datasets, accessible through a dedicated web interface, provide insights into human embryogenesis. We offer perspectives on the branching morphogenesis of human exocrine glands and unknown features of the development of neurovascular and skeletomuscular structures. These insights into human embryology have important implications for understanding craniofacial defects and neurological disorders and advancing diagnostic and therapeutic strategies.
Keywords: Tissue clearing; human embryo; iDISCO; light-sheet microscopy; oculomotor system; skull; vascular; virtual reality.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




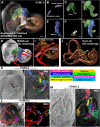

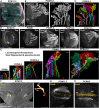


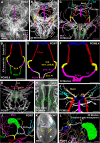
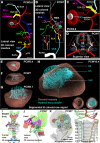
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

