Effect of whole-body cryotherapy versus placebo cryotherapy on joint pain induced by aromatase inhibitors in women with early stage breast cancer: a randomised clinical trial
- PMID: 38070928
- PMCID: PMC10729259
- DOI: 10.1136/bmjopen-2023-071756
Effect of whole-body cryotherapy versus placebo cryotherapy on joint pain induced by aromatase inhibitors in women with early stage breast cancer: a randomised clinical trial
Abstract
Introduction: Hormone therapy (HT) is a major adjuvant treatment for breast cancer. Despite their effectiveness, aromatase inhibitors can cause several side effects, including arthralgia in 35%-50% of patients. These side effects frequently lead to the premature discontinuation of HT. Whole-body cryotherapy (WBC) can be used for managing arthritic pain. The primary objective of this study will be to evaluate the effect of WBC on aromatase-induced joint pain, compared with placebo cryotherapy, in patients with hormone-dependent breast cancer receiving adjuvant aromatase inhibitors. The secondary objectives will be to evaluate WBC safety and its effect on analgesic consumption, HT adherence and quality of life.
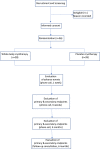
Methods and analysis: In this randomised, placebo-controlled, double-blinded clinical trial, 56 patients with aromatase inhibitor-induced joint pain and a Brief Pain Inventory-Short Form (BPI-SF) score ≥3 for the worst pain experienced in the previous week will be randomised into the WBC or placebo cryotherapy arm (10 sessions in each group). The primary outcome will be the BPI-SF score at week 6 post-treatment. The secondary outcomes will include the BPI-SF scores at months 3 and 6 post-treatment, the BPI-SF pain severity index and pain interference index, the Health Assessment Questionnaire score, the number of days of aromatase inhibitor treatment and analgesic consumption in the 15 days before the visits at week 6 and months 3 and 6 after cryotherapy. The incidence of adverse events will also be investigated.
Ethics and dissemination: Ethics approval was obtained from the Ethics Committee Est IV of Hospital Civil, Strasbourg, France. Protocol V.5 was approved in December 2022. The results will be disseminated in a peer-reviewed journal and presented at international congresses.
Trial registration number: NCT05315011.
Keywords: Adverse events; Breast tumours; PAIN MANAGEMENT; RHEUMATOLOGY.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
References
-
- Institut National du Cancer . Estimations Nationales de L’Incidence et de la Mortalité par cancer en France MéTropolitaine Entre 1990 et 2018. 2019. Available: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publica...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials