Metabolic labelling of a subpopulation of small extracellular vesicles using a fluorescent palmitic acid analogue
- PMID: 38072803
- PMCID: PMC10710952
- DOI: 10.1002/jev2.12392
Metabolic labelling of a subpopulation of small extracellular vesicles using a fluorescent palmitic acid analogue
Abstract
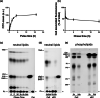
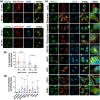
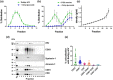
Exosomes are among the most puzzling vehicles of intercellular communication, but several crucial aspects of their biogenesis remain elusive, primarily due to the difficulty in purifying vesicles with similar sizes and densities. Here we report an effective methodology for labelling small extracellular vesicles (sEV) using Bodipy FL C16, a fluorescent palmitic acid analogue. In this study, we present compelling evidence that the fluorescent sEV population derived from Bodipy C16-labelled cells represents a discrete subpopulation of small exosomes following an intracellular pathway. Rapid cellular uptake and metabolism of Bodipy C16 resulted in the incorporation of fluorescent phospholipids into intracellular organelles specifically excluding the plasma membrane and ultimately becoming part of the exosomal membrane. Importantly, our fluorescence labelling method facilitated accurate quantification and characterization of exosomes, overcoming the limitations of nonspecific dye incorporation into heterogeneous vesicle populations. The characterization of Bodipy-labelled exosomes reveals their enrichment in tetraspanin markers, particularly CD63 and CD81, and in minor proportion CD9. Moreover, we employed nanoFACS sorting and electron microscopy to confirm the exosomal nature of Bodipy-labelled vesicles. This innovative metabolic labelling approach, based on the fate of a fatty acid, offers new avenues for investigating exosome biogenesis and functional properties in various physiological and pathological contexts.
Keywords: MVB; exosome biogenesis; exosomes; extracellular vesicles; lipid metabolism; sEV.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Akoumi, A. , Haffar, T. , Mousterji, M. , Kiss, R. S. , & Bousette, N. (2017). Palmitate mediated diacylglycerol accumulation causes endoplasmic reticulum stress, Plin2 degradation, and cell death in H9C2 cardiomyoblasts. Experimental Cell Research, 354(2), 85–94. 10.1016/j.yexcr.2017.03.032 - DOI - PubMed
-
- Coscia, C. , Parolini, I. , Sanchez, M. , Biffoni, M. , Boussadia, Z. , Zanetti, C. , Fiani, M. L. , & Sargiacomo, M. (2016). Generation, quantification, and tracing of metabolically labeled fluorescent exosomes. In Federico M., (ed.) Lentiviral vectors and exosomes as gene and protein delivery tools. pp. 217–235. Springer New York. - PubMed
-
- Escola, J. M. , Kleijmeer, M. J. , Stoorvogel, W. , Griffith, J. M. , Yoshie, O. , & Geuze, H. J. (1998). Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B‐lymphocytes. Journal of Biological Chemistry, 273(32), 20121–20127. 10.1074/jbc.273.32.20121 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

