In vivo safety and biodistribution profile of Klotho-enhanced human urine-derived stem cells for clinical application
- PMID: 38072946
- PMCID: PMC10712141
- DOI: 10.1186/s13287-023-03595-y
In vivo safety and biodistribution profile of Klotho-enhanced human urine-derived stem cells for clinical application
Abstract
Background: Urine-derived stem cells (UDSCs) can be easily isolated from urine and possess excellent stem cell characteristics, making them a promising source for cell therapeutics. Due to their kidney origin specificity, UDSCs are considered a superior therapeutic alternative for kidney diseases compared to other stem cells. To enhance the therapeutic potential of UDSCs, we developed a culture method that effectively boosts the expression of Klotho, a kidney-protective therapeutic factor. We also optimized the Good Manufacturing Practice (GMP) system to ensure stable and large-scale production of clinical-grade UDSCs from patient urine. In this study, we evaluated the in vivo safety and distribution of Klotho-enhanced UDSCs after intravenous administration in accordance with Good Laboratory Practice (GLP) regulations.
Methods: Mortality and general symptoms were continuously monitored throughout the entire examination period. We evaluated the potential toxicity of UDSCs according to the administration dosage and frequency using clinical pathological and histopathological analyses. We quantitatively assessed the in vivo distribution and retention period of UDSCs in major organs after single and repeated administration using human Alu-based qPCR analysis. We also conducted long-term monitoring for 26 weeks to assess the potential tumorigenicity.
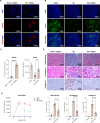
Results: Klotho-enhanced UDSCs exhibited excellent homing potential, and recovered Klotho expression in injured renal tissue. Toxicologically harmful effects were not observed in all mice after a single administration of UDSCs. It was also verified that repeated administration of UDSCs did not induce significant toxicological or immunological adverse effects in all mice. Single and repeated administrated UDSCs persisted in the blood and major organs for approximately 3 days and cleared in most organs, except the lungs, within 2 weeks. UDSCs that remained in the lungs were cleared out in approximately 4-5 weeks. There were no significant differences according to the variation of sex and administration frequency. The tumors were found in the intravenous administration group but they were confirmed to be non-human origin. Based on these results, it was clarified that UDSCs have no tumorigenic potential.
Conclusions: Our results demonstrate that Klotho-enhanced UDSCs can be manufactured as cell therapeutics through an optimized GMP procedure, and they can be safely administered without causing toxicity and tumorigenicity.
Keywords: Distribution; GMP; Klotho; Safety; Toxicity; Tumorigenicity; Urine-derived stem cells (UDSCs).
© 2023. The Author(s).
Conflict of interest statement
The authors affiliated with Affiliation 1 and Affiliation 2 are employees of EHLBio Co. Ltd. and EHLCell clinic, with Hong-Ki Lee serving as the CEO of both companies. They declare no competing financial interests, such as holding stocks or any other financial involvement related to the content of this paper. This study was conducted with complete scientific integrity and independence.
Figures





References
-
- Kang HS, Choi SH, Kim BS, Choi JY, Park GB, Kwon TG, et al. Advanced properties of urine derived stem cells compared to adipose tissue derived stem cells in terms of cell proliferation, immune modulation and multi differentiation. J Korean Med Sci. 2015;30(12):1764–1776. doi: 10.3346/jkms.2015.30.12.1764. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

