Adipsin inhibits Irak2 mitochondrial translocation and improves fatty acid β-oxidation to alleviate diabetic cardiomyopathy
- PMID: 38072993
- PMCID: PMC10712050
- DOI: 10.1186/s40779-023-00493-5
Adipsin inhibits Irak2 mitochondrial translocation and improves fatty acid β-oxidation to alleviate diabetic cardiomyopathy
Abstract
Background: Diabetic cardiomyopathy (DCM) causes the myocardium to rely on fatty acid β-oxidation for energy. The accumulation of intracellular lipids and fatty acids in the myocardium usually results in lipotoxicity, which impairs myocardial function. Adipsin may play an important protective role in the pathogenesis of DCM. The aim of this study is to investigate the regulatory effect of Adipsin on DCM lipotoxicity and its molecular mechanism.
Methods: A high-fat diet (HFD)-induced type 2 diabetes mellitus model was constructed in mice with adipose tissue-specific overexpression of Adipsin (Adipsin-Tg). Liquid chromatography-tandem mass spectrometry (LC-MS/MS), glutathione-S-transferase (GST) pull-down technique, Co-immunoprecipitation (Co-IP) and immunofluorescence colocalization analyses were used to investigate the molecules which can directly interact with Adipsin. The immunocolloidal gold method was also used to detect the interaction between Adipsin and its downstream modulator.
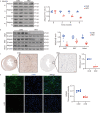
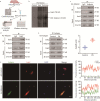
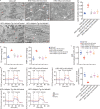
Results: The expression of Adipsin was significantly downregulated in the HFD-induced DCM model (P < 0.05). Adipose tissue-specific overexpression of Adipsin significantly improved cardiac function and alleviated cardiac remodeling in DCM (P < 0.05). Adipsin overexpression also alleviated mitochondrial oxidative phosphorylation function in diabetic stress (P < 0.05). LC-MS/MS analysis, GST pull-down technique and Co-IP studies revealed that interleukin-1 receptor-associated kinase-like 2 (Irak2) was a downstream regulator of Adipsin. Immunofluorescence analysis also revealed that Adipsin was co-localized with Irak2 in cardiomyocytes. Immunocolloidal gold electron microscopy and Western blotting analysis indicated that Adipsin inhibited the mitochondrial translocation of Irak2 in DCM, thus dampening the interaction between Irak2 and prohibitin (Phb)-optic atrophy protein 1 (Opa1) on mitochondria and improving the structural integrity and function of mitochondria (P < 0.05). Interestingly, in the presence of Irak2 knockdown, Adipsin overexpression did not further alleviate myocardial mitochondrial destruction and cardiac dysfunction, suggesting a downstream role of Irak2 in Adipsin-induced responses (P < 0.05). Consistent with these findings, overexpression of Adipsin after Irak2 knockdown did not further reduce the accumulation of lipids and their metabolites in the cardiac myocardium, nor did it enhance the oxidation capacity of cardiomyocytes expose to palmitate (PA) (P < 0.05). These results indicated that Irak2 may be a downstream regulator of Adipsin.
Conclusions: Adipsin improves fatty acid β-oxidation and alleviates mitochondrial injury in DCM. The mechanism is related to Irak2 interaction and inhibition of Irak2 mitochondrial translocation.
Keywords: Diabetic cardiomyopathy; Fatty acid β-oxidation; Mitochondrial function; Mitochondrial translocation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Comment in
-
Interleukin-1 receptor associated kinase 2 is a functional downstream regulator of complement factor D that controls mitochondrial fitness in diabetic cardiomyopathy.Mil Med Res. 2024 Jan 3;11(1):1. doi: 10.1186/s40779-023-00506-3. Mil Med Res. 2024. PMID: 38167325 Free PMC article. No abstract available.
-
Alleviating mitochondrial dysfunction in diabetic cardiomyopathy through the Adipsin and Irak2 pathways.Mil Med Res. 2024 Feb 1;11(1):11. doi: 10.1186/s40779-024-00513-y. Mil Med Res. 2024. PMID: 38303084 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

