Survival in macrophages induces enhanced virulence in Cryptococcus
- PMID: 38073033
- PMCID: PMC10826345
- DOI: 10.1128/msphere.00504-23
Survival in macrophages induces enhanced virulence in Cryptococcus
Abstract
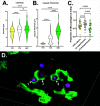
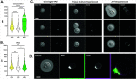
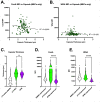
Cryptococcus is a ubiquitous environmental fungus and frequent colonizer of human lungs. Colonization can lead to diverse outcomes, from clearance to long-term colonization to life-threatening meningoencephalitis. Regardless of the outcome, the process starts with an encounter with phagocytes. Using the zebrafish model of this infection, we have noted that cryptococcal cells first spend time inside macrophages before they become capable of pathogenic replication and dissemination. What "licensing" process takes place during this initial encounter, and how are licensed cryptococcal cells different? To address this, we isolated cryptococcal cells after phagocytosis by cultured macrophages and found these macrophage-experienced cells to be markedly more virulent in both zebrafish and mouse models. Despite producing a thick polysaccharide capsule, they were still subject to phagocytosis by macrophages in the zebrafish. Analysis of antigenic cell wall components in these licensed cells demonstrated that components of mannose and chitin are more available for staining than they are in culture-grown cells or cells with capsule production induced in vitro. Cryptococcus is capable of exiting or transferring between macrophages in vitro, raising the likelihood that this fungus alternates between intracellular and extracellular life during growth in the lungs. Our results raise the possibility that intracellular life has its advantages over time, and phagocytosis-induced alteration in mannose and chitin exposure is one way that makes subsequent rounds of phagocytosis more beneficial to the fungus.IMPORTANCECryptococcosis begins in the lungs and can ultimately travel through the bloodstream to cause devastating infection in the central nervous system. In the zebrafish model, small amounts of cryptococcus inoculated into the bloodstream are initially phagocytosed and become far more capable of dissemination after they exit macrophages. Similarly, survival in the mouse lung produces cryptococcal cell types with enhanced dissemination. In this study, we have evaluated how phagocytosis changes the properties of Cryptococcus during pathogenesis. Macrophage-experienced cells (MECs) become "licensed" for enhanced virulence. They out-disseminate culture-grown cells in the fish and out-compete non-MECs in the mouse lung. Analysis of their cell surface demonstrates that MECs have increased availability of cell wall components mannose and chitin substances involved in provoking phagocytosis. These findings suggest how Cryptococcus might tune its cell surface to induce but survive repeated phagocytosis during early pathogenesis in the lung.
Keywords: Cryptococcus; capsule; dissemination; macrophage; phagocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Perfect JR. 2015. Cryptococcosis (Cryptococcus neoformans and Cryptococcus gatti), p 2934–2948. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas and Bennett’s principles and practice of infectious diseases, 8th ed. Elsevier Saunders, New York, NY.
-
- Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, Paul-Satyaseela M, Kim KS, Kwon-Chung KJ. 2004. Cryptococcal yeast cells invade the central nervous system via tanscellular penetration of the blood-brain barrier. Infect Immun 72:4985–4995. doi:10.1128/IAI.72.9.4985-4995.2004 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
