Matrix Metalloproteinase-7 in Urinary Extracellular Vesicles Identifies Rapid Disease Progression in Autosomal Dominant Polycystic Kidney Disease
- PMID: 38073039
- PMCID: PMC10914202
- DOI: 10.1681/ASN.0000000000000277
Matrix Metalloproteinase-7 in Urinary Extracellular Vesicles Identifies Rapid Disease Progression in Autosomal Dominant Polycystic Kidney Disease
Abstract
Significance statement: There is an unmet need for biomarkers of disease progression in autosomal dominant polycystic kidney disease (ADPKD). This study investigated urinary extracellular vesicles (uEVs) as a source of such biomarkers. Proteomic analysis of uEVs identified matrix metalloproteinase 7 (MMP-7) as a biomarker predictive of rapid disease progression. In validation studies, MMP-7 was predictive in uEVs but not in whole urine, possibly because uEVs are primarily secreted by tubular epithelial cells. Indeed, single-nucleus RNA sequencing showed that MMP-7 was especially increased in proximal tubule and thick ascending limb cells, which were further characterized by a profibrotic phenotype. Together, these data suggest that MMP-7 is a biologically plausible and promising uEV biomarker for rapid disease progression in ADPKD.
Background: In ADPKD, there is an unmet need for early markers of rapid disease progression to facilitate counseling and selection for kidney-protective therapy. Our aim was to identify markers for rapid disease progression in uEVs.
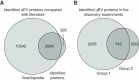
Methods: Six paired case-control groups ( n =10-59/group) of cases with rapid disease progression and controls with stable disease were formed from two independent ADPKD cohorts, with matching by age, sex, total kidney volume, and genetic variant. Candidate uEV biomarkers were identified by mass spectrometry and further analyzed using immunoblotting and an ELISA. Single-nucleus RNA sequencing of healthy and ADPKD tissue was used to identify the cellular origin of the uEV biomarker.
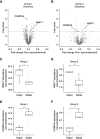
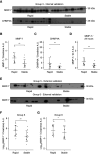
Results: In the discovery proteomics experiments, the protein abundance of MMP-7 was significantly higher in uEVs of patients with rapid disease progression compared with stable disease. In the validation groups, a significant >2-fold increase in uEV-MMP-7 in patients with rapid disease progression was confirmed using immunoblotting. By contrast, no significant difference in MMP-7 was found in whole urine using ELISA. Compared with healthy kidney tissue, ADPKD tissue had significantly higher MMP-7 expression in proximal tubule and thick ascending limb cells with a profibrotic phenotype.
Conclusions: Among patients with ADPKD, rapid disease progressors have higher uEV-associated MMP-7. Our findings also suggest that MMP-7 is a biologically plausible biomarker for more rapid disease progression.
Trial registration: ClinicalTrials.gov NCT02497521.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
R.T. Gansevoort reports Consultancy: AstraZeneca, Bayer, Galapagos, Mironid, and Sanofi-Genzyme; Research Funding: AstraZeneca, Abbvie, Bayer, Galapagos, Otsuka Pharmaceuticals, Roche, and Sanofi-Genzyme; Honoraria: Galapagos, Mironid, and Otsuka Pharmaceuticals; and Advisory or Leadership Role: Editor of
Figures






References
-
- Gansevoort RT Arici M Benzing T, et al. . Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016;31(3):337–348. doi:10.1093/ndt/gfv456 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

