Effectiveness of Bio-K+ for the prevention of Clostridioides difficile infection: Stepped-wedge cluster-randomized controlled trial
- PMID: 38073551
- PMCID: PMC11007362
- DOI: 10.1017/ice.2023.169
Effectiveness of Bio-K+ for the prevention of Clostridioides difficile infection: Stepped-wedge cluster-randomized controlled trial
Abstract
Objective: To evaluate the impact of administering probiotics to prevent Clostridioides difficile infection (CDI) among patients receiving therapeutic antibiotics.
Design: Stepped-wedge cluster-randomized trial between September 1, 2016, and August 31, 2019.
Setting: This study was conducted in 4 acute-care hospitals across an integrated health region.
Patients: Hospitalized patients, aged ≥55 years.
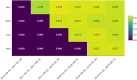
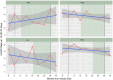
Methods: Patients were given 2 probiotic capsules daily (Bio-K+, Laval, Quebec, Canada), containing 50 billion colony-forming units of Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2. We measured hospital-acquired CDI (HA-CDI) and the number of positive C. difficile tests per 10,000 patient days as well as adherence to administration of Bio-K+ within 48 and 72 hours of antibiotic administration. Mixed-effects generalized linear models, adjusted for influenza admissions and facility characteristics, were used to evaluate the impact of the intervention on outcomes.
Results: Overall adherence of Bio-K+ administration ranged from 76.9% to 84.6% when stratified by facility and periods. Rates of adherence to administration within 48 and 72 hours of antibiotic treatment were 60.2% -71.4% and 66.7%-75.8%, respectively. In the adjusted analysis, there was no change in HA-CDI (incidence rate ratio [IRR], 0.92; 95% confidence interval [CI], 0.68-1.23) or C. difficile positivity rate (IRR, 1.05; 95% CI, 0.89-1.24). Discharged patients may not have received a complete course of Bio-K+. Our hospitals had a low baseline incidence of HA-CDI. Patients who did not receive Bio-K+ may have differential risks of acquiring CDI, introducing selection bias.
Conclusions: Hospitals considering probiotics as a primary prevention strategy should consider the baseline incidence of HA-CDI in their population and timing of probiotics relative to the start of antimicrobial administration.
Conflict of interest statement
J.L. reports funding from the Alberta Innovates’ Partnership for Research and Innovation in the Health System (PRIHS), M.S.I Foundation of Alberta, Canadian Institute of Health Research, and the Government of Canada Vanier Canada Graduate Scholarship. D.S. reports funding from Alberta Innovates’ Partnership for Research and Innovation in the Health System (PRIHS), membership on the Alberta Health Services Provincial Antimicrobial Stewardship Committee and the Calgary Zone Antimicrobial Stewardship Committee. B.M. reports grant funding from the Royal College of Physicians and Surgeons of Canada Professional Development Grant Project, Canadian Institutes of Health Research. B.M. also reports being a Physician Consultant for Medical Panels for Alberta’s Worker’s Compensation, has received Honoraria for IDSA ID Week Highlights Lecture from AVIR Pharma, is the President and Forum Representative for the Alberta Medical Association Infectious Diseases Section and the President of the Alberta Society for Infectious Diseases. T.L. reports funding from the Alberta Innovates’ Partnership for Research and Innovation in the Health System (PRIHS), grants or contracts for per case funding from Seres Therapeutics, Rebiotix, Finch Therapeutics, Summit PLC, Vedanta Biosciences, Crestone, MGB Biopharma and from the Canadian Institute for Health Research. T.L. reports attendance fees from Seres Therapeutics Advisory Board and consulting fess from MGB Biopharma. Additionally, T.L. reports travel support from Bio-K+ International Advisory Board to attend 1 meeting (October 18, 2022) to present the study data. J.C. reports funding from the Alberta Innovates’ Partnership for Research and Innovation in the Health System (PRIHS) in support of this study. J.C. reports accommodations and airfare from bioMerieux Canada to attend and speak at a symposium in 2022 on antimicrobial resistance cohosted by the University of Toronto and bioMerieux Canada. He is a member of the Cochrane Collaboration and works as an Infectious Diseases Consultant at Alberta Health Services, Calgary, Canada.
Figures


References
-
- Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare-facility–associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011;32:387–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

