Prevention of infections and fever to improve outcome in older patients with acute stroke (PRECIOUS): a randomised, open, phase III, multifactorial, clinical trial with blinded outcome assessment
- PMID: 38074444
- PMCID: PMC10698669
- DOI: 10.1016/j.lanepe.2023.100782
Prevention of infections and fever to improve outcome in older patients with acute stroke (PRECIOUS): a randomised, open, phase III, multifactorial, clinical trial with blinded outcome assessment
Abstract
Background: Infections and fever after stroke are associated with poor functional outcome or death. We assessed whether prophylactic treatment with anti-emetic, antibiotic, or antipyretic medication would improve functional outcome in older patients with acute stroke.
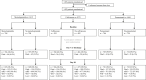
Methods: We conducted an international, 2∗2∗2-factorial, randomised, controlled, open-label trial with blinded outcome assessment in patients aged 66 years or older with acute ischaemic stroke or intracerebral haemorrhage and a score on the National Institutes of Health Stroke Scale ≥ 6. Patients were randomly allocated (1:1) to metoclopramide (oral, rectal, or intravenous; 10 mg thrice daily) vs. no metoclopramide, ceftriaxone (intravenous; 2000 mg once daily) vs. no ceftriaxone, and paracetamol (oral, rectal, or intravenous; 1000 mg four times daily) vs. no paracetamol, started within 24 h after symptom onset and continued for four days. All participants received standard of care. The target sample size was 3800 patients. The primary outcome was the score on the modified Rankin Scale (mRS) at 90 days analysed with ordinal logistic regression and reported as an adjusted common odds ratio (an acOR < 1 suggests benefit and an acOR > 1 harm). This trial is registered (ISRCTN82217627).
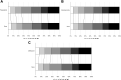
Findings: From April 2016 through June 2022, 1493 patients from 67 European sites were randomised to metoclopramide (n = 704) or no metoclopramide (n = 709), ceftriaxone (n = 594) or no ceftriaxone (n = 482), and paracetamol (n = 706) or no paracetamol (n = 739), of whom 1471 were included in the intention-to-treat analysis. Prophylactic use of study medication did not significantly alter the primary outcome at 90 days: metoclopramide vs. no metoclopramide (adjusted common odds ratio [acOR], 1.01; 95% CI 0.81-1.25), ceftriaxone vs. no ceftriaxone (acOR 0.99; 95% CI 0.77-1.27), paracetamol vs. no paracetamol (acOR 1.19; 95% CI 0.96-1.47). The study drugs were safe and not associated with an increased incidence of serious adverse events.
Interpretation: We observed no sign of benefit of prophylactic use of metoclopramide, ceftriaxone, or paracetamol during four days in older patients with a moderately severe to severe acute stroke.
Funding: This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No: 634809.
Keywords: Fever; Infection; Intracerebral haemorrhage; Ischaemic stroke; Pneumonia; Stroke.
© 2023 The Author(s).
Conflict of interest statement
LJW, BZ, IA, LC, IKJ, JeD, GN, HM, SR, SC, PC, SFTMdB, RP, KK, BC: none related. JCdJ, WMS, HR, JaD and MM report grants from the European Union, all paid to their institution. PMB reports having received grants from the UK National Institute of Health Research, and fees as consultant from CoMind, DiaMedica, Phagenesis and Roche. DvdB reports having received research grants from the European Union, The Netherlands for Health Research and Development, ItsMe Foundation, AMC Foundation and Roche; none related. AHA reports research grants from Boehringer Ingelheim, lectures fee from Abbvie, BMS/Pfizer, Novartis, Roche and Teva and participation in Advisory Board for Lundbeck, Abbvie and MSD; none related. AC reports grants from the European Union and Lombardy Region, for research paid to his institution, and fees as consultant or lecturer from Alexion Pharma, Daiichi Sanky, and Italfarmaco. JK reports lecturer fees from Boehringer Ingelheim, Pfizer and Servier, and travel grants from Boehringer Ingelheim and Servier, none related. SP received research support from BMS/Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, European Union, German Federal Joint Committee Innovation Fund, and German Federal Ministry of Education and Research, Helena Laboratories and Werfen as well as speakers’ honoraria/consulting fees from Alexion, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS/Pfizer, Daiichi Sankyo, Portola, and Werfen (all outside the submitted work). GT reports grants from the European Union, German Research Foundation, German Federal Ministry of Education and Research, German Innovation Fund for research paid to his institution, and fees as consultant or lecturer from Acandis, Alexion, Amarin, Bayer, Boehringer Ingelheim, Daiichi Sanky, BristolMyersSqibb/Pfizer, and Stryker. HBvdW reports having received grants from the European Union, the Dutch Heart Foundation, and Stryker for research, and funding for consultancy from Bayer and TargED, all paid to his institution.
Figures




References
-
- Greer D.M., Funk S.E., Reaven N.L., Ouzounelli M., Uman G.C. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39(11):3029–3035. - PubMed
-
- Ringelstein E.B., Chamorro A., Kaste M., et al. ESO Stroke Unit Certification Committee. European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke. 2013;44(3):828–840. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

