Metabolic and senescence characteristics associated with the immune microenvironment in ovarian cancer
- PMID: 38075052
- PMCID: PMC10702973
- DOI: 10.3389/fendo.2023.1265525
Metabolic and senescence characteristics associated with the immune microenvironment in ovarian cancer
Abstract
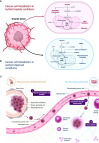
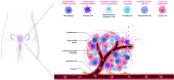
Ovarian cancer is a highly malignant gynecological cancer influenced by the immune microenvironment, metabolic reprogramming, and cellular senescence. This review provides a comprehensive overview of these characteristics. Metabolic reprogramming affects immune cell function and tumor growth signals. Cellular senescence in immune and tumor cells impacts anti-tumor responses and therapy resistance. Targeting immune cell metabolism and inducing tumor cell senescence offer potential therapeutic strategies. However, challenges remain in identifying specific targets and biomarkers. Understanding the interplay of these characteristics can lead to innovative therapeutic approaches. Further research is needed to elucidate mechanisms, validate strategies, and improve patient outcomes in ovarian cancer.
Keywords: immune microenvironment; metabolism; ovarian cancer; senescence; therapeutic strategies.
Copyright © 2023 Xiong, Fu, Huang, Wang, Jin, Wan, Huang and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet (2008) 371(9609):303–14. doi: 10.1016/S0140-6736(08)60167-1 - DOI - PubMed

