Synthesis of Fluorinated Amines: A Personal Account
- PMID: 38075451
- PMCID: PMC10704584
- DOI: 10.1021/acsorginorgau.3c00029
Synthesis of Fluorinated Amines: A Personal Account
Abstract
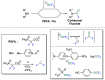
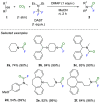
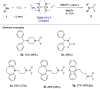
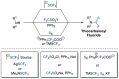
This Account highlights the recent contributions made by our laboratory in the development of novel strategies to synthesize fluorinated amines. These strategies allow the practitioner to efficiently access carbamoyl fluorides, thiocarbamoyl fluorides as well as trifluoromethylamines using CO2 or CS2 as benign C1 sources. In addition, a novel N(SCF3)CF3 moiety was synthesized. Noteworthy, we demonstrated that this reagent could also be used in radical- or electrophilic-based trifluoromethylthiolation reactions.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


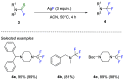
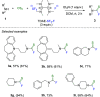
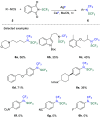
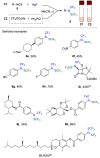
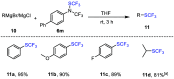
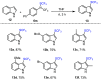
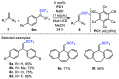
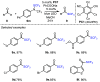
References
-
- Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe G., Leroux F., Eds.; Elsevier Science: London, 2018; pp 459–518.
-
- Kirsch P. In Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2013.
-
- Leo A.; Hansch C.; Elkins D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. 10.1021/cr60274a001. - DOI
-
- Linclau B.; Wang Z.; Compain G.; Paumelle V.; Fontenelle C. Q.; Wells N.; Weymouth-Wilson A. Investigating the Influence of (Deoxy)fluorination on the Lipophilicity of Non-UV-Active Fluorinated Alkanols and Carbohydrates by a New log P Determination Method. Angew. Chem. Int., Ed. 2016, 55, 674–678. 10.1002/anie.201509460. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
