Quantitative detection of microRNA-21 in vivo using in situ assembled photoacoustic and SERS nanoprobes
- PMID: 38075660
- PMCID: PMC10699575
- DOI: 10.1039/d3sc04371a
Quantitative detection of microRNA-21 in vivo using in situ assembled photoacoustic and SERS nanoprobes
Abstract
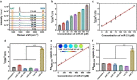
Accurately quantifying microRNA levels in vivo is of great importance for cancer staging and prognosis. However, the low abundance of microRNAs and interference from the complex tumor microenvironment usually limit the real-time quantification of microRNAs in vivo. Herein, for the first time, we develop an ultrasensitive microRNA (miR)-21 activated ratiometric nanoprobe for quantification of the miR-21 concentration in vivo without signal amplification as well as dynamic tracking of its distribution. The core-satellite nanoprobe by miR-21 triggered in situ self-assembly was built on nanogapped gold nanoparticles (AuNNP probe) and gold nanoparticles (AuNP probe). The AuNP probe generated a photoacoustic (PA) signal and ratiometric SERS signal with the variation of miR-21, whereas the AuNNP probe served as an internal standard, enabling ratiometric SERS imaging of miR-21. The absolute concentration of miR-21 in MCF-7 tumor-bearing mice was quantified to be 83.8 ± 24.6 pM via PA and ratiometric SERS imaging. Our strategy provides a powerful approach for the quantitative detection of microRNAs in vivo, providing a reference for the clinical treatment of cancer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

