Subthalamic nucleus shows opposite functional connectivity pattern in Huntington's and Parkinson's disease
- PMID: 38075949
- PMCID: PMC10699743
- DOI: 10.1093/braincomms/fcad282
Subthalamic nucleus shows opposite functional connectivity pattern in Huntington's and Parkinson's disease
Abstract
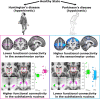
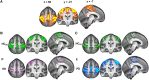
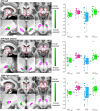
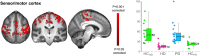
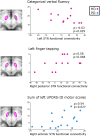
Huntington's and Parkinson's disease are two movement disorders representing mainly opposite states of the basal ganglia inhibitory function. Despite being an integral part of the cortico-subcortico-cortical circuitry, the subthalamic nucleus function has been studied at the level of detail required to isolate its signal only through invasive studies in Huntington's and Parkinson's disease. Here, we tested whether the subthalamic nucleus exhibited opposite functional signatures in early Huntington's and Parkinson's disease. We included both movement disorders in the same whole-brain imaging study, and leveraged ultra-high-field 7T MRI to achieve the very fine resolution needed to investigate the smallest of the basal ganglia nuclei. Eleven of the 12 Huntington's disease carriers were recruited at a premanifest stage, while 16 of the 18 Parkinson's disease patients only exhibited unilateral motor symptoms (15 were at Stage I of Hoehn and Yahr off medication). Our group comparison interaction analyses, including 24 healthy controls, revealed a differential effect of Huntington's and Parkinson's disease on the functional connectivity at rest of the subthalamic nucleus within the sensorimotor network, i.e. an opposite effect compared with their respective age-matched healthy control groups. This differential impact in the subthalamic nucleus included an area precisely corresponding to the deep brain stimulation 'sweet spot'-the area with maximum overall efficacy-in Parkinson's disease. Importantly, the severity of deviation away from controls' resting-state values in the subthalamic nucleus was associated with the severity of motor and cognitive symptoms in both diseases, despite functional connectivity going in opposite directions in each disorder. We also observed an altered, opposite impact of Huntington's and Parkinson's disease on functional connectivity within the sensorimotor cortex, once again with relevant associations with clinical symptoms. The high resolution offered by the 7T scanner has thus made it possible to explore the complex interplay between the disease effects and their contribution on the subthalamic nucleus, and sensorimotor cortex. Taken altogether, these findings reveal for the first time non-invasively in humans a differential, clinically meaningful impact of the pathophysiological process of these two movement disorders on the overall sensorimotor functional connection of the subthalamic nucleus and sensorimotor cortex.
Keywords: Huntington’s; Parkinson’s; differential effect; functional connectivity; subthalamic nucleus.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. - PubMed
-
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. - PubMed
-
- Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18(6):595–604. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
