Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives
- PMID: 38076401
- PMCID: PMC10700415
- DOI: 10.1016/j.cdnut.2023.102026
Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives
Abstract
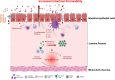
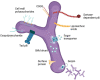
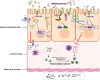
The intestinal tight junction (TJ) barrier is a crucial defense mechanism that prevents the passage of intestinal content into the intestinal wall, tissue, and systemic circulation. A compromised intestinal TJ barrier has been identified as a significant factor in inflammatory bowel disease (IBD), necrotizing enterocolitis, and other gut-related inflammatory conditions. Recent studies have revealed the importance of the probiotic bacterial strains of Bifidobacterium in protecting against intestinal inflammation and IBD pathogenesis via the regulation of intestinal TJ barrier function. Numerous species and strains of Bifidobacterium have been found to regulate TJ proteins and the signaling pathways responsible for maintaining intestinal barrier integrity and permeability. In this review, we provide a summary of recent studies that highlight the regulatory role of Bifidobacterium species and the strain effect on the intestinal TJ barrier. We also discuss the intracellular mechanisms involved in Bifidobacterium modulation of the intestinal barrier and the potential therapeutic efficacy of targeting the barrier function to regulate intestinal inflammation.
Keywords: Bifidobacterium; cytokines; gastrointestinal inflammation; gut microbiota; intestinal tight junction barrier.
© 2023 The Authors.
Conflict of interest statement
The authors report no conflict of interest.
Figures
References
-
- Ma T.Y., Nighot P., Al-Sadi R. In: Physiology of the Gastrointestinal Tract. 6th ed. Said H.M., editor. Elsevier; 2018. Tight junctions and the intestinal barrier; pp. 587–639. Available from: - DOI
-
- Buschmann M.M., Shen L., Rajapakse H., Raleigh D.R., Wang Y., Wang Y., et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell. 2013;24(19):3056–3068. doi: 10.1091/mbc.E12-09-0688. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

