Efficacy and safety of MP1032 plus standard-of-care compared to standard-of-care in hospitalised patients with COVID-19: a multicentre, randomised double-blind, placebo-controlled phase 2a trial
- PMID: 38076629
- PMCID: PMC10704330
- DOI: 10.1016/j.lanepe.2023.100810
Efficacy and safety of MP1032 plus standard-of-care compared to standard-of-care in hospitalised patients with COVID-19: a multicentre, randomised double-blind, placebo-controlled phase 2a trial
Abstract
Background: SARS-CoV-2 infections still have a significant impact on the global population. The existing vaccinations have contributed to reducing the severe disease courses, decreasing hospitalisations, and lowering the mortality rate. However, due to the variability of COVID-19 symptoms, the emergence of new variants and the uneven global distribution of vaccines there is still a great need for new therapy options. One promising approach is provided by host-directed therapies. We assessed here the efficacy and safety of MP1032, a host-directed anti-viral/anti-inflammatory drug in hospitalised patients with moderate to severe COVID-19.
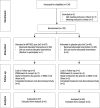
Methods: In a randomised, double-blind, placebo-controlled, Phase IIa study, patients were randomised 2:1 to receive either 300 mg MP032 bid + Standard-of-Care (SoC) or placebo bid + SoC for 28 days. Eligible patients were ≥18 years old, tested positive for SARS-CoV-2, and had moderate to severe COVID-19 symptoms. The study spanned 20 sites in six countries (Bulgaria, France, Hungary, Italy, Romania, Spain), assessing disease progression according the NIAID scale as the primary outcome on day 14. Secondary objectives included disease progression (day 28), disease resolution (days 14 and 28), mortality rate, COVID-19 related parameters and safety. Exposure-response analyses were performed, linking MP1032 to COVID-19 biomarkers (eGFR, D-dimer).
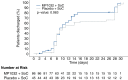
Findings: 132 patients were enrolled to receive MP1032 + SoC (n = 87) or placebo + SoC (n = 45). The patients were all white or Caucasian with a mean (median) age of 60.5 (63) years. Overall, only 10 patients were vaccinated, 5 in each group. No significant risk difference of disease progression could be detected between groups on both day 14 (9.8% MP1032 vs. 11.6% placebo) and day 28 with MH common risk differences of -0.276% (95% CI, -11.634 to 11.081; p = 0.962) and 1.722% (95% CI, -4.576 to 8.019; p = 0.592), respectively.The treatment with MP1032 + SoC was safe and well-tolerated. Overall, 182 TEAEs including 10 SAEs were reported in 53.5% (46/86) of patients of the verum group and in 57.8% (26/45) of patients of the placebo group; the SAEs occurred in 5.8% (5/86) and 6.7% (3/45) of verum and placebo patients, respectively. None of the SAEs was considered as related.
Interpretation: Despite the study's limitation in size and the variation in concurrent SoCs, these findings warrant further investigation of MP1032 as a host-directed anti-viral drug candidate.
Funding: The study was funded by the COVID-19 Horizon Europe work programme and MetrioPharm AG.
Keywords: Anti-infective; Anti-inflammatory; Covid-19; Host-directed therapy; Pandemic preparedness.
© 2023 The Author(s).
Conflict of interest statement
The authors’ affiliations are as follows: P.S., A.K., S.S., B.L. M.N., I.S. are employees of MetrioPharm Deutschland GmbH. W.B. is employer, co-founder, and CSO of MetrioPharm AG and CMO of MetrioPharm GmbH. K.O., C.D. and T.L. are employees of Saarmetrics GmbH and are also members of Department of Clinical Pharmacy of University of Saarland, Germany.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Sep 9;21(1):772. doi: 10.1186/s13063-020-04588-5. Trials. 2020. PMID: 32907638 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
Cited by
-
Efficacy of MP1032 in hospitalised patients with COVID-19- authors' reply.Lancet Reg Health Eur. 2024 Feb 2;38:100857. doi: 10.1016/j.lanepe.2024.100857. eCollection 2024 Mar. Lancet Reg Health Eur. 2024. PMID: 38328414 Free PMC article. No abstract available.
-
Efficacy of MP1032 in hospitalised patients with COVID-19.Lancet Reg Health Eur. 2024 Feb 2;38:100856. doi: 10.1016/j.lanepe.2024.100856. eCollection 2024 Mar. Lancet Reg Health Eur. 2024. PMID: 38328410 Free PMC article. No abstract available.
-
[Syphilis in surgery-Safe diagnosis and correct treatment].Chirurgie (Heidelb). 2024 Nov;95(11):906-913. doi: 10.1007/s00104-024-02114-w. Epub 2024 Jun 10. Chirurgie (Heidelb). 2024. PMID: 38858242 Review. German.
References
-
- World Health Organization (WHO) COVID-19 weekly epidemiological update. 2023. p. 15.
-
- Lamers M.M., Haagmans B.L. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20(5):270–284. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

