Synthesis and pharmacological properties of coumarin-chalcones
- PMID: 38076708
- PMCID: PMC10698004
- DOI: 10.1016/j.mex.2023.102488
Synthesis and pharmacological properties of coumarin-chalcones
Abstract
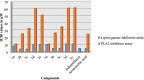
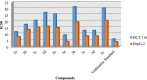
New chalcones (2a-e) were prepared by Claisen-Schmidt condensation from 3-acetyl-4-hydroxycoumarin, which was used as a key intermediate in this synthesis. However, we can easily obtain compounds (3a-e) by refluxing chalcone (2a-e) with 4-hydroxycoumarin in the presence of ammonium acetate and glacial acetic acid. Multinuclear NMR (1H and 13C), IR and elemental analysis characterized the structure of the final compound. The antibacterial activity of the obtained products against various bacterial strains was tested in vitro. The antioxidant properties of the same synthesized compounds were also studied using DPPH (2,2-diphenyl-1-trinitrophenylhydrazine) and hydroxyl radical scavenging tests. Furthemore a study was conducted to highlight the nature of the effects produced by screening 2a-e and 3a-e products on colon cancer cell lines (HCT-116) and hepatocellular carcinoma cell lines (HepG-2). Good cytotoxic activity against standard vinblastine was observed for compound 3a. •3-acetyl-4-hydroxycoumarin as a simple coumarinic ketone was modified to coumarins-bonded chalcones.•Modification was performed through two steps reaction.•Final products exhibited free radical scavenging activity and Good cytotoxic.
Keywords: Anti-inflammatory activity and antiproliferative activity; Antibacterial; Antioxidant; Coumarin; Coumarin chalcones hybrids; Synthesis of coumarin -bonded chalcone derivatives via two steps reaction.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Viegas-Junior C., Danuello A., da Silva Bolzani V., Barreiro E.J., Fraga C.A. Curr. Med. Chem. 2007;14:1829–1852. - PubMed
-
- Sandhu S., Bansal Y., Silakari O., Bansal G. Bioorg. Med. Chem. 2014;22:3806–3814. - PubMed
-
- Gediya L.K., Njar V.C. Expert Opin. Drug Discov. 2009;4:1099–1111. - PubMed
-
- Dasari B., Jimmidi R., Arya P. Eur. J. Med. Chem. 2015;94:497–508. - PubMed
-
- Chen X., Decker M. Curr. Med. Chem. 2013;20:1673–1685. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

