This is a preprint.
A (Sub)field Guide to Quality Control in Hippocampal Subfield Segmentation on Highresolution T2-weighted MRI
- PMID: 38076964
- PMCID: PMC10705396
- DOI: 10.1101/2023.11.29.568895
A (Sub)field Guide to Quality Control in Hippocampal Subfield Segmentation on Highresolution T2-weighted MRI
Update in
-
A (sub)field guide to quality control in hippocampal subfield segmentation on high-resolution T2-weighted MRI.Hum Brain Mapp. 2024 Oct 15;45(15):e70004. doi: 10.1002/hbm.70004. Hum Brain Mapp. 2024. PMID: 39450914 Free PMC article. Review.
Abstract
Inquiries into properties of brain structure and function have progressed due to developments in magnetic resonance imaging (MRI). To sustain progress in investigating and quantifying neuroanatomical details in vivo, the reliability and validity of brain measurements are paramount. Quality control (QC) is a set of procedures for mitigating errors and ensuring the validity and reliability of brain measurements. Despite its importance, there is little guidance on best QC practices and reporting procedures. The study of hippocampal subfields in vivo is a critical case for QC because of their small size, inter-dependent boundary definitions, and common artifacts in the MRI data used for subfield measurements. We addressed this gap by surveying the broader scientific community studying hippocampal subfields on their views and approaches to QC. We received responses from 37 investigators spanning 10 countries, covering different career stages, and studying both healthy and pathological development and aging. In this sample, 81% of researchers considered QC to be very important or important, and 19% viewed it as fairly important. Despite this, only 46% of researchers reported on their QC processes in prior publications. In many instances, lack of reporting appeared due to ambiguous guidance on relevant details and guidance for reporting, rather than absence of QC. Here, we provide recommendations for correcting errors to maximize reliability and minimize bias. We also summarize threats to segmentation accuracy, review common QC methods, and make recommendations for best practices and reporting in publications. Implementing the recommended QC practices will collectively improve inferences to the larger population, as well as have implications for clinical practice and public health.
Figures

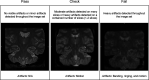
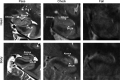
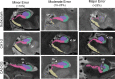

References
-
- Adler D. H., Wisse L. E. M., Ittyerah R., Pluta J. B., Ding S.-L., Xie L., Wang J., Kadivar S., Robinson J. L., Schuck T., Trojanowski J. Q., Grossman M., Detre J. A., Elliott M. A., Toledo J. B., Liu W., Pickup S., Miller M. I., Das S. R., … Yushkevich P. A. (2018). Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proceedings of the National Academy of Sciences, 115(16), 4252–4257. 10.1073/pnas.1801093115 - DOI - PMC - PubMed
-
- Alfaro-Almagro F., Jenkinson M., Bangerter N. K., Andersson J. L. R., Griffanti L., Douaud G., Sotiropoulos S. N., Jbabdi S., Hernandez-Fernandez M., Vallee E., Vidaurre D., Webster M., McCarthy P., Rorden C., Daducci A., Alexander D. C., Zhang H., Dragonu I., Matthews P. M., … Smith S. M. (2018). Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage, 166, 400–424. 10.1016/j.neuroimage.2017.10.034 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
