This is a preprint.
AMP-activated protein kinase is necessary for Treg cell functional adaptation to microenvironmental stress
- PMID: 38076988
- PMCID: PMC10705412
- DOI: 10.1101/2023.11.29.568904
AMP-activated protein kinase is necessary for Treg cell functional adaptation to microenvironmental stress
Update in
-
AMPK is necessary for Treg functional adaptation to microenvironmental stress during malignancy and viral pneumonia.J Clin Invest. 2025 Mar 18;135(9):e179572. doi: 10.1172/JCI179572. eCollection 2025 May 1. J Clin Invest. 2025. PMID: 40100289 Free PMC article.
Abstract
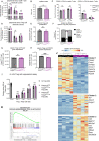
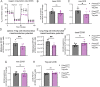
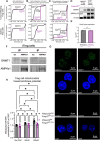
CD4+FOXP3+ regulatory T (Treg) cells maintain self-tolerance, suppress the immune response to cancer, and protect against tissue injury in the lung and other organs. Treg cells require mitochondrial metabolism to exert their function, but how Treg cells adapt their metabolic programs to sustain and optimize their function during an immune response occurring in a metabolically stressed microenvironment remains unclear. Here, we tested whether Treg cells require the energy homeostasis-maintaining enzyme AMP-activated protein kinase (AMPK) to adapt to metabolically aberrant microenvironments caused by malignancy or lung injury, finding that AMPK is dispensable for Treg cell immune-homeostatic function but is necessary for full Treg cell function in B16 melanoma tumors and during acute lung injury caused by influenza virus pneumonia. AMPK-deficient Treg cells had lower mitochondrial mass and exhibited an impaired ability to maximize aerobic respiration. Mechanistically, we found that AMPK regulates DNA methyltransferase 1 to promote transcriptional programs associated with mitochondrial function in the tumor microenvironment. In the lung during viral pneumonia, we found that AMPK sustains metabolic homeostasis and mitochondrial activity. Induction of DNA hypomethylation was sufficient to rescue mitochondrial mass in AMPK-deficient Treg cells, linking DNA methylation with AMPK function and mitochondrial metabolism. These results define AMPK as a determinant of Treg cell adaptation to metabolic stress and offer potential therapeutic targets in cancer and tissue injury.
Conflict of interest statement
Competing Interest Statement: NM is currently an employee and owns stock in Vertex Pharmaceuticals. BDS holds United States Patent No. US 10,905,706 B2, Compositions and Methods to Accelerate Resolution of Acute Lung Inflammation, and serves on the Scientific Advisory Board of Zoe Biosciences. The other authors have no competing interests to declare.
Figures



References
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M., Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155, 1151–1164 (1995). - PubMed
Publication types
Grants and funding
- R01 HL153122/HL/NHLBI NIH HHS/United States
- K08 HL159356/HL/NHLBI NIH HHS/United States
- S10 OD011996/OD/NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- P01 HL154998/HL/NHLBI NIH HHS/United States
- T32 AI083216/AI/NIAID NIH HHS/United States
- F31 HL162490/HL/NHLBI NIH HHS/United States
- R01 HL149883/HL/NHLBI NIH HHS/United States
- T32 GM144295/GM/NIGMS NIH HHS/United States
- F32 HL162418/HL/NHLBI NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
