This is a preprint.
Estradiol Mediates Greater Germinal Center Responses to Influenza Vaccination in Female than Male Mice
- PMID: 38077071
- PMCID: PMC10705292
- DOI: 10.1101/2023.11.27.568847
Estradiol Mediates Greater Germinal Center Responses to Influenza Vaccination in Female than Male Mice
Update in
-
Estradiol mediates greater germinal center responses to influenza vaccination in female than male mice.mBio. 2024 Apr 10;15(4):e0032624. doi: 10.1128/mbio.00326-24. Epub 2024 Mar 5. mBio. 2024. PMID: 38441028 Free PMC article.
Abstract
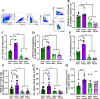
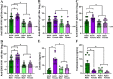
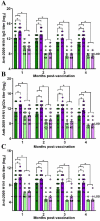
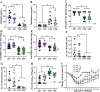
Adult females of reproductive ages develop greater antibody responses to inactivated influenza vaccine (IIV) than males. How sex, age, and sex steroid changes impact B cells and durability of IIV-induced immunity and protection over 4-months post-vaccination (mpv) was analyzed. Vaccinated adult females had greater germinal center (GC) B cell and plasmablast frequencies in lymphoid tissues, higher neutralizing antibody responses 1-4 mpv, and better protection against live H1N1 challenge than adult males. Aged mice, regardless of sex, had reduced B cell frequencies, less durable antibody responses, and inferior protection after challenge than adult mice, which correlated with diminished estradiol among aged females. To confirm that greater IIV-induced immunity was caused by sex hormones, four core genotype (FCG) mice were used, in which the testes determining gene, Sry, was deleted from ChrY and transferred to Chr3, to separate gonadal sex (i.e., ovaries or testes) from sex chromosome complement (i.e., XX or XY complement). Vaccinated, gonadal female FCG mice (XXF and XYF) had greater numbers of B cells, higher antiviral antibody titers, and reduced pulmonary virus titers following live H1N1 challenge than gonadal FCG males (XYM and XXM). To establish that lower estradiol concentrations cause diminished immunity, adult and aged females received either a placebo or estradiol replacement therapy prior to IIV. Estradiol replacement significantly increased IIV-induced antibody responses and reduced morbidity after the H1N1 challenge among aged females. These data highlight that estradiol is a targetable mechanism mediating greater humoral immunity following vaccination among adult females.
Figures


References
-
- Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, Klimov A, Keitel WA, Nichol KL, Carr WW, Treanor JJ. 2008. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med 168:2405–14. - PubMed
-
- Živković I, Petrović R, Arsenović-Ranin N, Petrušić V, Minić R, Bufan B, Popović O, Leposavić G. 2018. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals 52:18–24. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
