This is a preprint.
A Mouse Model of the Protease Activated Receptor 4 (PAR4) Pro310Leu Variant has Reduced Platelet Reactivity
- PMID: 38077081
- PMCID: PMC10705540
- DOI: 10.1101/2023.12.01.569075
A Mouse Model of the Protease Activated Receptor 4 (PAR4) Pro310Leu Variant has Reduced Platelet Reactivity
Update in
-
A mouse model of the protease-activated receptor 4 Pro310Leu variant has reduced platelet reactivity.J Thromb Haemost. 2024 Jun;22(6):1715-1726. doi: 10.1016/j.jtha.2024.03.004. Epub 2024 Mar 19. J Thromb Haemost. 2024. PMID: 38508397 Free PMC article.
Abstract
Background: Protease activated receptor 4 (PAR4) mediates thrombin signaling on platelets and other cells. Our recent structural studies demonstrated a single nucleotide polymorphism in extracellular loop 3 (ECL3), PAR4-P310L (rs2227376) leads to a hypo-reactive receptor.
Objectives: The goal of this study was to determine how the hypo-reactive PAR4 variant in ECL3 impacts platelet function in vivo using a novel knock-in mouse model (PAR4-322L).
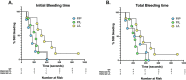
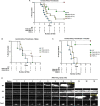
Methods: A point mutation was introduced into the PAR4 gene, F2rl3, via CRISPR/Cas9 to create PAR4-P322L, the mouse homolog to human PAR4-P310L. Platelet response to PAR4 activation peptide (AYPGKF), thrombin, ADP, and convulxin was monitored by αIIbβ3 integrin activation and P-selectin translocation using flow cytometry or platelet aggregation. In vivo responses were determined by the tail bleeding assay and the ferric chloride-induced carotid artery injury model.
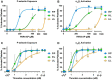
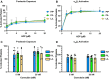
Results: PAR4-P/L and PAR4-L/L platelets had a reduced response to AYPGKF and thrombin measured by P-selectin translocation or αIIbβ3 activation. The response to ADP and convulxin was unchanged among genotypes. In addition, both PAR4-P/L and PAR4-L/L platelets showed a reduced response to thrombin in aggregation studies. There was an increase in the tail bleeding time for PAR4-L/L mice. The PAR4-P/L and PAR4-L/L mice both showed an extended time to arterial thrombosis.
Conclusions: PAR4-322L significantly reduced platelet responsiveness to AYPGKF and thrombin, which is in agreement with our previous structural and cell signaling studies. In addition, PAR4-322L had prolonged arterial thrombosis time. Our mouse model provides a foundation to further evaluate the role of PAR4 in other pathophysiological contexts.
Keywords: Protease activated receptor 4; mouse model; platelet function; single nucleotide polymorphisms (SNPs); thrombin receptor.
Conflict of interest statement
Authors conflict of interest statement The authors declare no conflicts of interest.
Figures


References
-
- Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev 2011; 25: 155–67. - PubMed
-
- Brass LF. Thrombin and Platelet Activation. Chest 2003; 124: 18S–25S. - PubMed
-
- Vu T-KH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991; 64: 1057–68. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
