Ceramides in peripheral arterial plaque lead to endothelial cell dysfunction
- PMID: 38077163
- PMCID: PMC10704331
- DOI: 10.1016/j.jvssci.2023.100181
Ceramides in peripheral arterial plaque lead to endothelial cell dysfunction
Abstract
Background: Peripheral arterial atheroprogression is increasingly prevalent, and is a risk factor for major limb amputations in individuals with risk factors such as diabetes. We previously demonstrated that bioactive lipids are significantly altered in arterial tissue of individuals with diabetes and advanced peripheral arterial disease.
Methods: Here we evaluated whether sphingolipid ceramide 18:1/16:0 (C16) is a cellular regulator in endothelial cells and peripheral tibial arterial tissue in individuals with diabetes.
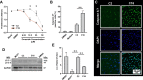
Results: We observed that C16 is the single most elevated ceramide in peripheral arterial tissue from below the knee in individuals with diabetes (11% increase, P < .05). C16 content in tibial arterial tissue positively correlates with sphingomyelin (SPM) content in patients with and without diabetes (r2 = 0.5, P < .005; r2 = 0.17, P < .05; respectively). Tibial arteries of individuals with diabetes demonstrated no difference in CERS6 expression (encoding ceramide synthase 6; the predominate ceramide synthesis enzyme), but higher SMPD expression (encoding sphingomyelin phosphodiesterase that catalyzes ceramide synthesis from sphingomyelins; P < .05). SMPD4, but not SMPD2, was particularly elevated in maximally diseased (Max) tibial arterial segments (P < .05). In vitro, exogenous C16 caused endothelial cells (HUVECs) to have decreased proliferation (P < .03), increased apoptosis (P < .003), and decreased autophagy (P < .008). Selective knockdown of SMPD2 and SMPD4 decreased native production of C16 (P < .01 and P < .001, respectively), but only knockdown of SMPD4 rescued cellular proliferation (P < .005) following exogenous supplementation with C16.
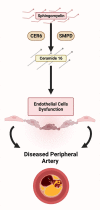
Conclusions: Our findings suggest that C16 is a tissue biomarker for peripheral arterial disease severity in the setting of diabetes, and can impact endothelial cell viability and function.
Clinical relevance: Peripheral arterial disease and its end-stage manifestation known as chronic limb-threatening ischemia (CLTI) represent ongoing prevalent and intricate medical challenges. Individuals with diabetes have a heightened risk of developing CLTI and experiencing its complications, including wounds, ulcers, and major amputations. In the present study, we conducted a comprehensive examination of the molecular lipid composition within arterial segments from individuals with CLTI, and with and without diabetes. Our investigations unveiled a striking revelation: the sphingolipid ceramide 18:1/16:0 emerged as the predominant ceramide species that was significantly elevated in the peripheral arterial intima below the knee in patients with diabetes. Moreover, this heightened ceramide presence is associated with a marked impairment of endothelial cell function and viability. Additionally, our study revealed a concurrent elevation in the expression of sphingomyelin phosphodiesterases, enzymes responsible for catalyzing ceramide synthesis from sphingomyelins, within maximally diseased arterial segments. These findings underscore the pivotal role of ceramides and their biosynthesis enzymes in the context of CLTI, offering new insights into potential therapeutic avenues for managing this challenging disease process.
Keywords: Ceramide; Diabetes; Endothelial dysfunction; Peripheral arterial disease; Sphingolipid; Sphingomyelinase.
Conflict of interest statement
None.
Figures



References
-
- White J.V., Conte M., Bradbury A., et al. Building a global alliance in vascular surgery. J Vasc Surg. 2019;70:663–664. - PubMed
-
- Nehler M.R., Duval S., Diao L., et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695.e2. - PubMed
-
- Fowkes F.G.R., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

