Aortic dissection detection and thrombus structure quantification using volumetric ultrasound, histology, and scanning electron microscopy
- PMID: 38077164
- PMCID: PMC10709059
- DOI: 10.1016/j.jvssci.2023.100105
Aortic dissection detection and thrombus structure quantification using volumetric ultrasound, histology, and scanning electron microscopy
Abstract
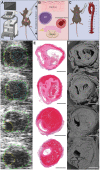
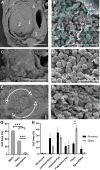
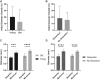
Aortic dissection occurs when a weakened portion of the intima tears, and a separation of layers propagates along the aortic wall to form a false lumen filled with active blood flow or intramural thrombus. The unpredictable nature of aortic dissection formation and need for immediate intervention leaves limited serial human image data to study the formation and morphological changes that follow dissection. We used volumetric ultrasound examination, histology, and scanning electron microscopy (SEM) to examine intramural thrombi at well-defined timepoints after dissection occurs in apolipoprotein E-deficient mice infused with angiotensin II (n = 71). Stratification of red blood cell (RBC) morphologies (biconcave, intermediate biconcave, intermediate polyhedrocyte, and polyhedrocyte) in the thrombi with scanning electron microscopy (n = 5) was used to determine degree of thrombus deposition/contraction. Very few biconcave RBCs (1.2 ± 0.6%) were in the thrombi, and greater amounts of intermediate biconcave RBCs (25.8 ± 6.7%) were located in the descending thoracic portion of the dissection while more polyhedrocytes (14.6 ± 5.1%) and fibrin (42.3 ± 4.5%; P < .05) were found in the distal suprarenal aorta. Thrombus deposition likely plays some role in patient outcomes, and this multimodality technique can help investigate thrombus deposition and characteristics in experimental animal models and human tissue samples.
Keywords: Aortic dissection; Intramural thrombus formation; Scanning electron microscopy.
© 2023 by the Society for Vascular Surgery. Published by Elsevier Inc.
Figures







Similar articles
-
Polyhedral erythrocytes in intracoronary thrombus and their association with reperfusion in myocardial infarction.Clin Res Cardiol. 2019 Aug;108(8):950-962. doi: 10.1007/s00392-019-01425-x. Epub 2019 Feb 1. Clin Res Cardiol. 2019. PMID: 30710262
-
Recruitment of labelled monocytes by experimental venous thrombi.Thromb Haemost. 2001 Jun;85(6):1018-24. Thromb Haemost. 2001. PMID: 11434678
-
Variations of dissection properties and mass fractions with thrombus age in human abdominal aortic aneurysms.J Biomech. 2014 Jan 3;47(1):14-23. doi: 10.1016/j.jbiomech.2013.10.027. Epub 2013 Oct 23. J Biomech. 2014. PMID: 24309621
-
Role of transesophageal echocardiography in dissection of the aorta and evaluation of degenerative aortic disease.Cardiol Clin. 1993 Aug;11(3):461-73. Cardiol Clin. 1993. PMID: 8402774 Review.
-
[Diagnostic goals in aortic dissection. Value of transthoracic and transesophageal echocardiography].Herz. 1992 Dec;17(6):321-37. Herz. 1992. PMID: 1483622 Review. German.
Cited by
-
Functional cardiac consequences of β-adrenergic stress-induced injury in the mdx mouse model of Duchenne muscular dystrophy.bioRxiv [Preprint]. 2024 Apr 20:2024.04.15.589650. doi: 10.1101/2024.04.15.589650. bioRxiv. 2024. Update in: Dis Model Mech. 2024 Oct 1;17(10):dmm050852. doi: 10.1242/dmm.050852. PMID: 38659739 Free PMC article. Updated. Preprint.
-
Functional cardiac consequences of β-adrenergic stress-induced injury in a model of Duchenne muscular dystrophy.Dis Model Mech. 2024 Oct 1;17(10):dmm050852. doi: 10.1242/dmm.050852. Epub 2024 Oct 9. Dis Model Mech. 2024. PMID: 39268580 Free PMC article.
-
Deconstructing fibrin(ogen) structure.J Thromb Haemost. 2025 Feb;23(2):368-380. doi: 10.1016/j.jtha.2024.10.024. Epub 2024 Nov 12. J Thromb Haemost. 2025. PMID: 39536819 Review.
References
-
- Levy D., Goyal A., Grigorova Y., Farci F., Le J.K. StatPearls. StatPearls Publishing; 2022. Aortic dissection. - PubMed
-
- Nienaber C.A., Kische S., Rousseau H., Eggerbrecht H., Rehders T.C., Kundt G., et al. Endovascular repair of type B aortic dissection. Circ Cardiovasc Interv. 2013;6:407–416. - PubMed
LinkOut - more resources
Full Text Sources

