Spatial architecture of high-grade glioma reveals tumor heterogeneity within distinct domains
- PMID: 38077210
- PMCID: PMC10699851
- DOI: 10.1093/noajnl/vdad142
Spatial architecture of high-grade glioma reveals tumor heterogeneity within distinct domains
Abstract
Background: High-grade gliomas (HGGs) are aggressive primary brain cancers with poor response to standard regimens, driven by immense heterogeneity. In isocitrate dehydrogenase (IDH) wild-type HGG (glioblastoma, GBM), increased intratumoral heterogeneity is associated with more aggressive disease.
Methods: Spatial technologies can dissect complex heterogeneity within the tumor ecosystem by preserving cellular organization in situ. We employed GeoMx digital spatial profiling, CosMx spatial molecular imaging, Xenium in situ mapping and Visium spatial gene expression in experimental and validation patient cohorts to interrogate the transcriptional landscape in HGG.
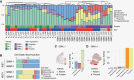
Results: Here, we construct a high-resolution molecular map of heterogeneity in GBM and IDH-mutant patient samples to investigate the cellular communities that compose HGG. We uncovered striking diversity in the tumor landscape and degree of spatial heterogeneity within the cellular composition of the tumors. The immune distribution was diverse between samples, however, consistently correlated spatially with distinct tumor cell phenotypes, validated across tumor cohorts. Reconstructing the tumor architecture revealed two distinct niches, one composed of tumor cells that most closely resemble normal glial cells, associated with microglia, and the other niche populated by monocytes and mesenchymal tumor cells.
Conclusions: This primary study reveals high levels of intratumoral heterogeneity in HGGs, associated with a diverse immune landscape within spatially localized regions.
Keywords: brain cancer; glioma; immune microenvironment; sequencing; spatial transcriptomics.
© The Author(s) 2023. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
S.A.B. received instrument support (GeoMx®) from NanoString Technologies as highlighted in the Acknowledgments section. A.P., D.S., L.Z., and Y.L. are employees and stockholders of NanoString Technologies.
Figures





References
-
- van den Bent MJ, Brandes AA, Taphoorn MJB, et al. . Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2013;31(3):344–350. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
