Endosomal trafficking inhibitor EGA can control TLR7-mediated IFNα expression by human plasmacytoid dendritic cells
- PMID: 38077311
- PMCID: PMC10704457
- DOI: 10.3389/fimmu.2023.1202197
Endosomal trafficking inhibitor EGA can control TLR7-mediated IFNα expression by human plasmacytoid dendritic cells
Abstract
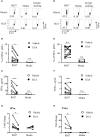
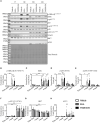
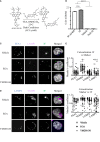
Plasmacytoid dendritic cells (pDC) are the major producer of type 1 IFN in response to TLR7 agonists. Aberrant TLR7 activation and type 1 IFN expression by pDCs are linked to the pathogenesis of certain types of autoimmune diseases, including systemic lupus erythematosus (SLE). This study investigated the underlying mechanisms for TLR7-mediated cytokine expression by pDCs using a late endosome trafficking inhibitor, EGA (4-bromobenzaldehyde N-(2,6-dimethylphenyl) semicarbazone). We found that EGA treatment decreased IFNα expression by pDCs stimulated with imiquimod (R837), single-stranded RNA40, and influenza virus. EGA also decreased TNFα expression and secretion by R837-stimulated pDCs. Mechanistically, EGA treatment decreased phosphorylation of IKKα/β, STAT1, and p38, and prolonged degradation of IκBα. Furthermore, EGA treatment decreased the colocalization of 3F, a substituted adenine TLR7 agonist, with LAMP1+ compartments in pDCs. EGA was also capable of diminishing IFNα expression by SLE pDCs treated with R837 or live PR8/A/34 influenza viruses. Therefore, we concluded that trafficking of TLR7 agonists to LAMP1+ compartments is important for IFNα expression by pDCs. Data from this study support additional examinations of the potential benefits of EGA in treating type 1 IFN-associated inflammatory diseases in the future.
Keywords: EGA; TLR7; endosome trafficking; innate immunity; nucleic acid; plasmacytoid dendritic cells; proinflammatory cytokine; type 1 interferon.
Copyright © 2023 Wiest, Baert, Gu, Gayler, Ham, Gorvel, Keddis, Griffing, Joo, Gorvel, Billadeau, Kane and Oh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

