Accelerating development of engineered T cell therapies in the EU: current regulatory framework for studying multiple product versions and T2EVOLVE recommendations
- PMID: 38077331
- PMCID: PMC10704912
- DOI: 10.3389/fimmu.2023.1280826
Accelerating development of engineered T cell therapies in the EU: current regulatory framework for studying multiple product versions and T2EVOLVE recommendations
Abstract
To accelerate the development of Advanced Therapy Medicinal Products (ATMPs) for patients suffering from life-threatening cancer with limited therapeutic options, regulatory approaches need to be constantly reviewed, evaluated and adjusted, as necessary. This includes utilizing science and risk-based approaches to mitigate and balance potential risks associated with early clinical research and a more flexible manufacturing paradigm. In this paper, T2EVOLVE an Innovative Medicine Initiative (IMI) consortium explores opportunities to expedite the development of CAR and TCR engineered T cell therapies in the EU by leveraging tools within the existing EU regulatory framework to facilitate an iterative and adaptive learning approach across different product versions with similar design elements or based on the same platform technology. As understanding of the linkage between product quality attributes, manufacturing processes, clinical efficacy and safety evolves through development and post licensure, opportunities are emerging to streamline regulatory submissions, optimize clinical studies and extrapolate data across product versions reducing the need to perform duplicative studies. It is worth noting that this paper is focusing on CAR- and TCR-engineered T cell therapies but the concepts may be applied more broadly to engineered cell therapy products (e.g., CAR NK cell therapy products).
Keywords: advanced therapy medicinal products (ATMP); clinical development; engineered T cell therapies; multiple product candidates; parent-child approach.
Copyright © 2023 Ammar, Schapitz, Luu, Hudecek, Meyer, Taps, Schröder, Ivics, Sanges, Franz, Koehl, Negre, Johanna and Awigena-Cook.
Conflict of interest statement
Author DA was employed by Astellas. Author IS was employed by Bayer Vital GmbH. Author MM was employed by Immatics Biotechnologies GmbH. Author TT was employed by Century Therapeutics. Author BS was employed by Miltenyi Biotec B.V. & Co. KG. Author HN was employed by Institut de Recherches Internationales Servier, Gif-sur-Yvette, France. Author JA-C was employed by Bristol Myers Squibb. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






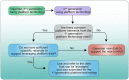
References
-
- US Food and Drug Administration . Studying Multiple Versions of a Cellular of Gene Therapy Product in an Early-Phase Clinical Trial, Guidance for Industry (2022). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents... (Accessed 1 Aug 2023).
-
- Taps T. The parent–child IND approach: an interpretation of FDA’s guidance on studying multiple versions of a cellular or gene therapy product in an early-phase clinical trial. Cell Gene Ther Insights (2022) 8(11):1581–6. doi: 10.18609/cgti.2022.229 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

