The molecular mechanism of actions and clinical utilities of tumor infiltrating lymphocytes in gastrointestinal cancers: a comprehensive review and future prospects toward personalized medicine
- PMID: 38077386
- PMCID: PMC10704251
- DOI: 10.3389/fimmu.2023.1298891
The molecular mechanism of actions and clinical utilities of tumor infiltrating lymphocytes in gastrointestinal cancers: a comprehensive review and future prospects toward personalized medicine
Abstract
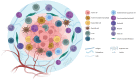
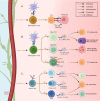
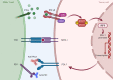
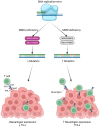
Gastrointestinal (GI) cancers remain a significant global health burden, accounting for a substantial number of cases and deaths. Regrettably, the inadequacy of dependable biomarkers hinders the precise forecasting of patient prognosis and the selection of appropriate therapeutic sequencing for individuals with GI cancers, leading to suboptimal outcomes for numerous patients. The intricate interplay between tumor-infiltrating lymphocytes (TILs) and the tumor immune microenvironment (TIME) has been shown to be a pivotal determinant of response to anti-cancer therapy and consequential clinical outcomes across a multitude of cancer types. Therefore, the assessment of TILs has garnered global interest as a promising prognostic biomarker in oncology, with the potential to improve clinical decision-making substantially. Moreover, recent discoveries in immunotherapy have progressively changed the landscape of cancer treatment and significantly prolonged the survival of patients with advanced cancers. Nonetheless, the response rate remains constrained within solid tumor sufferers, even when TIL landscapes appear comparable, which calls for the development of our understanding of cellular and molecular cross-talk between TIME and tumor. Hence, this comprehensive review encapsulates the extant literature elucidating the TILs' underlying molecular pathogenesis, prognostic significance, and their relevance in the realm of immunotherapy for patients afflicted by GI tract cancers. Within this review, we demonstrate that the type, density, and spatial distribution of distinct TIL subpopulations carries pivotal implications for the prediction of anti-cancer treatment responses and patient survival. Furthermore, this review underscores the indispensable role of TILs in modulating therapeutic responses within distinct molecular subtypes, such as those characterized by microsatellite stability or programmed cell death ligand-1 expression in GI tract cancers. The review concludes by outlining future directions in TIL-based personalized medicine, including integrating TIL-based approaches into existing treatment regimens and developing novel therapeutic strategies that exploit the unique properties of TILs and their potential as a promising avenue for personalized cancer treatment.
Keywords: gastrointestinal tract cancer; immune infiltration; immune microenvironment; immunotherapy; tumor-infiltrating lymphocytes.
Copyright © 2023 Piroozkhah, Gholinezhad, Piroozkhah, Shams and Nazemalhosseini-Mojarad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Lymphocyte infiltration in breast cancer: A promising prognostic indicator.Medicine (Baltimore). 2024 Dec 6;103(49):e40845. doi: 10.1097/MD.0000000000040845. Medicine (Baltimore). 2024. PMID: 39654199 Free PMC article. Review.
-
Tumor-infiltrating lymphocytes in melanoma: from prognostic assessment to therapeutic applications.Front Immunol. 2024 Dec 6;15:1497522. doi: 10.3389/fimmu.2024.1497522. eCollection 2024. Front Immunol. 2024. PMID: 39712007 Free PMC article. Review.
-
The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes.Cancer Med. 2019 Dec;8(17):7207-7218. doi: 10.1002/cam4.2580. Epub 2019 Oct 11. Cancer Med. 2019. PMID: 31605439 Free PMC article.
-
Association of Germline Variants in Natural Killer Cells With Tumor Immune Microenvironment Subtypes, Tumor-Infiltrating Lymphocytes, Immunotherapy Response, Clinical Outcomes, and Cancer Risk.JAMA Netw Open. 2019 Sep 4;2(9):e199292. doi: 10.1001/jamanetworkopen.2019.9292. JAMA Netw Open. 2019. PMID: 31483464 Free PMC article.
-
Tumor-infiltrating lymphocytes as a prognostic and predictive factor for Melanoma.Expert Rev Mol Diagn. 2024 Apr;24(4):299-310. doi: 10.1080/14737159.2024.2312102. Epub 2024 Feb 5. Expert Rev Mol Diagn. 2024. PMID: 38314660 Free PMC article. Review.
Cited by
-
Applications and advances of multi-omics technologies in gastrointestinal tumors.Front Med (Lausanne). 2025 Jul 23;12:1630788. doi: 10.3389/fmed.2025.1630788. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40771479 Free PMC article. Review.
-
IL-2RG as a possible immunotherapeutic target in CRC predicting poor prognosis and regulated by miR-7-5p and miR-26b-5p.J Transl Med. 2024 May 8;22(1):439. doi: 10.1186/s12967-024-05251-2. J Transl Med. 2024. PMID: 38720389 Free PMC article.
-
Prognostic Impact of Klintrup-Mäkinen (KM) Score in Gastric Cancer and Its Association with Pathological Parameters.Medicina (Kaunas). 2025 Apr 13;61(4):715. doi: 10.3390/medicina61040715. Medicina (Kaunas). 2025. PMID: 40283006 Free PMC article.
-
Emerging Concepts in Immuno-Oncology: Insights from Natural Language Processing-Driven Co-Occurrence Analysis.ACS Omega. 2025 Jun 27;10(27):28587-28614. doi: 10.1021/acsomega.5c00693. eCollection 2025 Jul 15. ACS Omega. 2025. PMID: 40686960 Free PMC article. Review.
-
Tumor-infiltrating lymphocytes in cancer immunotherapy: from chemotactic recruitment to translational modeling.Front Immunol. 2025 May 22;16:1601773. doi: 10.3389/fimmu.2025.1601773. eCollection 2025. Front Immunol. 2025. PMID: 40475782 Free PMC article. Review.
References
-
- Ji X, Bu ZD, Yan Y, Li ZY, Wu AW, Zhang LH, et al. . The 8th edition of the American Joint Committee on Cancer tumor-node-metastasis staging system for gastric cancer is superior to the 7th edition: results from a Chinese mono-institutional study of 1663 patients. Gastric Cancer (2018) 21(4):643–52. doi: 10.1007/s10120-017-0779-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

