More rapid blood interferon α2 decline in fatal versus surviving COVID-19 patients
- PMID: 38077399
- PMCID: PMC10703045
- DOI: 10.3389/fimmu.2023.1250214
More rapid blood interferon α2 decline in fatal versus surviving COVID-19 patients
Abstract
Background: The clinical outcome of COVID-19 pneumonia is highly variable. Few biological predictive factors have been identified. Genetic and immunological studies suggest that type 1 interferons (IFN) are essential to control SARS-CoV-2 infection.
Objective: To study the link between change in blood IFN-α2 level and plasma SARS-Cov2 viral load over time and subsequent death in patients with severe and critical COVID-19.
Methods: One hundred and forty patients from the CORIMUNO-19 cohort hospitalized with severe or critical COVID-19 pneumonia, all requiring oxygen or ventilation, were prospectively studied. Blood IFN-α2 was evaluated using the Single Molecule Array technology. Anti-IFN-α2 auto-Abs were determined with a reporter luciferase activity. Plasma SARS-Cov2 viral load was measured using droplet digital PCR targeting the Nucleocapsid gene of the SARS-CoV-2 positive-strand RNA genome.
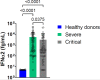
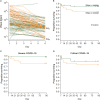
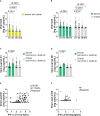
Results: Although the percentage of plasmacytoid dendritic cells was low, the blood IFN-α2 level was higher in patients than in healthy controls and was correlated to SARS-CoV-2 plasma viral load at entry. Neutralizing anti-IFN-α2 auto-antibodies were detected in 5% of patients, associated with a lower baseline level of blood IFN-α2. A longitudinal analysis found that a more rapid decline of blood IFN-α2 was observed in fatal versus surviving patients: mortality HR=3.15 (95% CI 1.14-8.66) in rapid versus slow decliners. Likewise, a high level of plasma SARS-CoV-2 RNA was associated with death risk in patients with severe COVID-19.
Conclusion: These findings could suggest an interest in evaluating type 1 IFN treatment in patients with severe COVID-19 and type 1 IFN decline, eventually combined with anti-inflammatory drugs.
Clinical trial registration: https://clinicaltrials.gov, identifiers NCT04324073, NCT04331808, NCT04341584.
Keywords: COVID-19; SARS-CoV-2; pneumonia; prospective study; type I interferon.
Copyright © 2023 Joly, Desjardins, Porcher, Péré, Bruneau, Zhang, Bastard, Cobat, Resmini, Lenoir, Savale, Lécuroux, Verstuyft, Roque-Afonso, Veyer, Baron, Resche-Rigon, Ravaud, Casanova, Le Grand, Hermine, Tharaux and Mariette.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

