Glutamine addiction and therapeutic strategies in pancreatic cancer
- PMID: 38077649
- PMCID: PMC10701242
- DOI: 10.4251/wjgo.v15.i11.1852
Glutamine addiction and therapeutic strategies in pancreatic cancer
Abstract
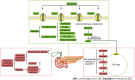
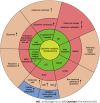
Pancreatic cancer remains one of the most lethal diseases worldwide owing to its late diagnosis, early metastasis, and poor prognosis. Because current therapeutic options are limited, there is an urgent need to investigate novel targeted treatment strategies. Pancreatic cancer faces significant metabolic challenges, principally hypoxia and nutrient deprivation, due to specific microenvironmental constraints, including an extensive desmoplastic stromal reaction. Pancreatic cancer cells have been shown to rewire their metabolism and energy production networks to support rapid survival and proliferation. Increased glucose uptake and glycolytic pathway activity during this process have been extensively described. However, growing evidence suggests that pancreatic cancer cells are glutamine addicted. As a nitrogen source, glutamine directly (or indirectly via glutamate conversion) contributes to many anabolic processes in pancreatic cancer, including amino acids, nucleobases, and hexosamine biosynthesis. It also plays an important role in redox homeostasis, and when converted to α-ketoglutarate, glutamine serves as an energy and anaplerotic carbon source, replenishing the tricarboxylic acid cycle intermediates. The present study aims to provide a comprehensive overview of glutamine metabolic reprogramming in pancreatic cancer, focusing on potential therapeutic approaches targeting glutamine metabolism in pancreatic cancer.
Keywords: Cancer treatment; Glutamine metabolism; Pancreatic cancer; Therapeutic strategies.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest.
Figures

References
-
- Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11. - PubMed
-
- Bott AJ, Shen J, Tonelli C, Zhan L, Sivaram N, Jiang YP, Yu X, Bhatt V, Chiles E, Zhong H, Maimouni S, Dai W, Velasquez S, Pan JA, Muthalagu N, Morton J, Anthony TG, Feng H, Lamers WH, Murphy DJ, Guo JY, Jin J, Crawford HC, Zhang L, White E, Lin RZ, Su X, Tuveson DA, Zong WX. Glutamine Anabolism Plays a Critical Role in Pancreatic Cancer by Coupling Carbon and Nitrogen Metabolism. Cell Rep. 2019;29:1287–1298.e6. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

