Heavy water coupling gel for short-wave infrared photoacoustic imaging
- PMID: 38078156
- PMCID: PMC10704084
- DOI: 10.1117/1.JBO.28.11.116001
Heavy water coupling gel for short-wave infrared photoacoustic imaging
Abstract
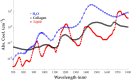
Significance: Changes in lipid, water, and collagen (LWC) content in tissue are associated with numerous medical abnormalities (cancer, atherosclerosis, and Alzheimer's disease). Standard imaging modalities are limited in resolution, specificity, and/or penetration for quantifying these changes. Short-wave infrared (SWIR) photoacoustic imaging (PAI) has the potential to overcome these challenges by exploiting the unique optical absorption properties of .
Aim: This study's aim is to harness SWIR PAI for mapping LWC changes in tissue. The focus lies in devising a reflection-mode PAI technique that surmounts current limitations related to SWIR light delivery.
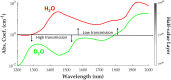
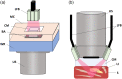
Approach: To enhance light delivery for reflection-mode SWIR PAI, we designed a deuterium oxide (, "heavy water") gelatin (HWG) interface for opto-acoustic coupling, intended to significantly improve light transmission above 1200 nm.
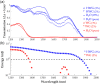
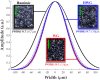
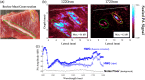
Results: HWG permits light delivery up to 1850 nm, which was not possible with water-based coupling ( light delivery up to 1350 nm). PAI using the HWG interface and the Visualsonics Vevo LAZR-X reveals a signal increase up to 24 dB at 1720 nm in lipid-rich regions.
Conclusions: By overcoming barriers related to light penetration, the HWG coupling interface enables accurate quantification/monitoring of biomarkers like LWC using reflection-mode PAI. This technological stride offers potential for tracking changes in chronic diseases (in vivo) and evaluating their responses to therapeutic interventions.
Keywords: cancer; collagen; heavy water; high resolution ultrasound; lipids; optoacoustic imaging; photoacoustic imaging and spectroscopy; short-wave infrared.
© 2023 The Authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

