Dysregulated iron homeostasis in dystrophin-deficient cardiomyocytes: correction by gene editing and pharmacological treatment
- PMID: 38078368
- PMCID: PMC10898935
- DOI: 10.1093/cvr/cvad182
Dysregulated iron homeostasis in dystrophin-deficient cardiomyocytes: correction by gene editing and pharmacological treatment
Abstract
Aims: Duchenne muscular dystrophy (DMD)-associated cardiomyopathy is a serious life-threatening complication, the mechanisms of which have not been fully established, and therefore no effective treatment is currently available. The purpose of the study was to identify new molecular signatures of the cardiomyopathy development in DMD.
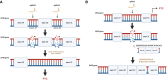
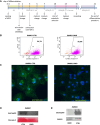
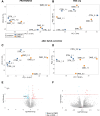
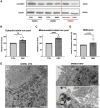
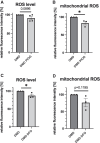
Methods and results: For modelling of DMD-associated cardiomyopathy, we prepared three pairs of isogenic control and dystrophin-deficient human induced pluripotent stem cell (hiPSC) lines. Two isogenic hiPSC lines were obtained by CRISPR/Cas9-mediated deletion of DMD exon 50 in unaffected cells generated from healthy donor and then differentiated into cardiomyocytes (hiPSC-CM). The latter were subjected to global transcriptomic and proteomic analyses followed by more in-depth investigation of selected pathway and pharmacological modulation of observed defects. Proteomic analysis indicated a decrease in the level of mitoNEET protein in dystrophin-deficient hiPSC-CM, suggesting alteration in iron metabolism. Further experiments demonstrated increased labile iron pool both in the cytoplasm and mitochondria, a decrease in ferroportin level and an increase in both ferritin and transferrin receptor in DMD hiPSC-CM. Importantly, CRISPR/Cas9-mediated correction of the mutation in the patient-derived hiPSC reversed the observed changes in iron metabolism and restored normal iron levels in cardiomyocytes. Moreover, treatment of DMD hiPSC-CM with deferoxamine (DFO, iron chelator) or pioglitazone (mitoNEET stabilizing compound) decreased the level of reactive oxygen species in DMD hiPSC-CM.
Conclusion: To our knowledge, this study demonstrated for the first time impaired iron metabolism in human DMD cardiomyocytes, and potential reversal of this effect by correction of DMD mutation or pharmacological treatment. This implies that iron overload-regulating compounds may serve as novel therapeutic agents in DMD-associated cardiomyopathy.
Keywords: CISD1; CRISPR/Cas9; Cardiomyocytes; Cardiomyopathy; DMD; Deferoxamine; Duchenne muscular dystrophy; Induced pluripotent stem cells; Iron overload; MitoNEET; Pioglitazone; hiPSC-CM.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared. This manuscript was handled by Deputy Editor Pasquale Maffia.
Figures



References
-
- Ishikawa Y, Miura T, Ishikawa Y, Aoyagi T, Ogata H, Hamada S, Minami R. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord 2011;21:47–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

