Endothelin signaling in development
- PMID: 38078652
- PMCID: PMC10753589
- DOI: 10.1242/dev.201786
Endothelin signaling in development
Abstract
Since the discovery of endothelin 1 (EDN1) in 1988, the role of endothelin ligands and their receptors in the regulation of blood pressure in normal and disease states has been extensively studied. However, endothelin signaling also plays crucial roles in the development of neural crest cell-derived tissues. Mechanisms of endothelin action during neural crest cell maturation have been deciphered using a variety of in vivo and in vitro approaches, with these studies elucidating the basis of human syndromes involving developmental differences resulting from altered endothelin signaling. In this Review, we describe the endothelin pathway and its functions during the development of neural crest-derived tissues. We also summarize how dysregulated endothelin signaling causes developmental differences and how this knowledge may lead to potential treatments for individuals with gene variants in the endothelin pathway.
Keywords: Auriculocondylar syndrome; Cardiovascular; Craniofacial; Enteric; G protein; Hirschsprung disease; Melanocyte.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



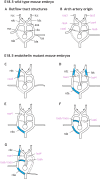
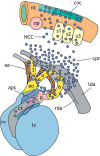

References
-
- Adameyko, I., Lallemend, F., Furlan, A., Zinin, N., Aranda, S., Kitambi, S. S., Blanchart, A., Favaro, R., Nicolis, S., Lübke, M.et al. (2012). Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139, 397-410. 10.1242/dev.065581 - DOI - PMC - PubMed
-
- Alexander, C., Zuniga, E., Blitz, I. L., Wada, N., Le Pabic, P., Javidan, Y., Zhang, T., Cho, K. W., Crump, J. G. and Schilling, T. F. (2011). Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development 138, 5135-5146. 10.1242/dev.067801 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

