Butyrate reduces adherent-invasive E. coli-evoked disruption of epithelial mitochondrial morphology and barrier function: involvement of free fatty acid receptor 3
- PMID: 38078655
- PMCID: PMC10730202
- DOI: 10.1080/19490976.2023.2281011
Butyrate reduces adherent-invasive E. coli-evoked disruption of epithelial mitochondrial morphology and barrier function: involvement of free fatty acid receptor 3
Abstract
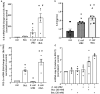
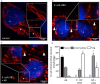
Gut bacteria provide benefits to the host and have been implicated in inflammatory bowel disease (IBD), where adherent-invasive E. coli (AIEC) pathobionts (e.g., strain LF82) are associated with Crohn's disease. E. coli-LF82 causes fragmentation of the epithelial mitochondrial network, leading to increased epithelial permeability. We hypothesized that butyrate would limit the epithelial mitochondrial disruption caused by E. coli-LF82. Human colonic organoids and the T84 epithelial cell line infected with E. coli-LF82 (MOI = 100, 4 h) showed a significant increase in mitochondrial network fission that was reduced by butyrate (10 mM) co-treatment. Butyrate reduced the loss of mitochondrial membrane potential caused by E. coli-LF82 and increased expression of PGC-1 mRNA, the master regulator of mitochondrial biogenesis. Metabolomics revealed that butyrate significantly altered E. coli-LF82 central carbon metabolism leading to diminished glucose uptake and increased succinate secretion. Correlating with preservation of mitochondrial network form/function, butyrate reduced E. coli-LF82 transcytosis across T84-cell monolayers. The use of the G-protein inhibitor, pertussis toxin, implicated GPCR signaling as critical to the effect of butyrate, and the free fatty acid receptor three (FFAR3, GPR41) agonist, AR420626, reproduced butyrate's effect in terms of ameliorating the loss of barrier function and reducing the mitochondrial fragmentation observed in E. coli-LF82 infected T84-cells and organoids. These data indicate that butyrate helps maintain epithelial mitochondrial form/function when challenged by E. coli-LF82 and that this occurs, at least in part, via FFAR3. Thus, loss of butyrate-producing bacteria in IBD in the context of pathobionts would contribute to loss of epithelial mitochondrial and barrier functions that could evoke disease and/or exaggerate a low-grade inflammation.
Keywords: Pathobiont; T84 cells; gut epithelium; mitochondrial dynamics; organoid; permeability.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
