BLK positively regulates TLR/IL-1R signaling by catalyzing TOLLIP phosphorylation
- PMID: 38078859
- PMCID: PMC10711807
- DOI: 10.1083/jcb.202302081
BLK positively regulates TLR/IL-1R signaling by catalyzing TOLLIP phosphorylation
Abstract
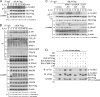
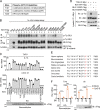
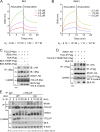
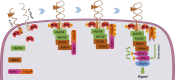
TLR/IL-1R signaling plays a critical role in sensing various harmful foreign pathogens and mounting efficient innate and adaptive immune responses, and it is tightly controlled by intracellular regulators at multiple levels. In particular, TOLLIP forms a constitutive complex with IRAK1 and sequesters it in the cytosol to maintain the kinase in an inactive conformation under unstimulated conditions. However, the underlying mechanisms by which IRAK1 dissociates from TOLLIP to activate TLR/IL-1R signaling remain obscure. Herein, we show that BLK positively regulates TLR/IL-1R-mediated inflammatory response. BLK-deficient mice produce less inflammatory cytokines and are more resistant to death upon IL-1β challenge. Mechanistically, BLK is preassociated with IL1R1 and IL1RAcP in resting cells. IL-1β stimulation induces heterodimerization of IL1R1 and IL1RAcP, which further triggers BLK autophosphorylation at Y309. Activated BLK directly phosphorylates TOLLIP at Y76/86/152 and further promotes TOLLIP dissociation from IRAK1, thereby facilitating TLR/IL-1R-mediated signal transduction. Overall, these findings highlight the importance of BLK as an active regulatory component in TLR/IL-1R signaling.
© 2023 Li et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






Similar articles
-
Leishmania donovani Exploits Tollip, a Multitasking Protein, To Impair TLR/IL-1R Signaling for Its Survival in the Host.J Immunol. 2018 Aug 1;201(3):957-970. doi: 10.4049/jimmunol.1800062. Epub 2018 Jun 15. J Immunol. 2018. PMID: 29907707
-
PINK1 positively regulates IL-1β-mediated signaling through Tollip and IRAK1 modulation.J Neuroinflammation. 2012 Dec 17;9:271. doi: 10.1186/1742-2094-9-271. J Neuroinflammation. 2012. PMID: 23244239 Free PMC article.
-
Tollip Inhibits ST2 Signaling in Airway Epithelial Cells Exposed to Type 2 Cytokines and Rhinovirus.J Innate Immun. 2020;12(1):103-115. doi: 10.1159/000497072. Epub 2019 Mar 29. J Innate Immun. 2020. PMID: 30928973 Free PMC article.
-
IRAK1: a critical signaling mediator of innate immunity.Cell Signal. 2008 Feb;20(2):269-76. doi: 10.1016/j.cellsig.2007.08.009. Epub 2007 Aug 23. Cell Signal. 2008. PMID: 17890055 Review.
-
Interleukin Receptor Associated Kinase 1 Signaling and Its Association with Cardiovascular Diseases.Rev Cardiovasc Med. 2022 Mar 12;23(3):97. doi: 10.31083/j.rcm2303097. Rev Cardiovasc Med. 2022. PMID: 35345264 Free PMC article. Review.
Cited by
-
Deciphering the Role of LncRNAs in Osteoarthritis: Inflammatory Pathways Unveiled.J Inflamm Res. 2024 Sep 20;17:6563-6581. doi: 10.2147/JIR.S489682. eCollection 2024. J Inflamm Res. 2024. PMID: 39318993 Free PMC article. Review.
-
Yuyang Decoction Regulates Macrophage Polarization and Repairs the Intestinal Mucosal Barrier via the TLRs/Tollip Signaling Pathway.J Inflamm Res. 2025 Aug 4;18:10499-10518. doi: 10.2147/JIR.S515902. eCollection 2025. J Inflamm Res. 2025. PMID: 40787257 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 2021YFD1800300/National Key Research and Development Program of China
- 32202781/National Natural Science Foundation of China
- Fundamental Research Funds for the Central Universities
- 21ZD3NA001/Key Technologies Research and Development Program of Gansu Province
- 22JR5RA034/Natural Science Foundation of Gansu Province
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

