Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial
- PMID: 38078870
- PMCID: PMC10714284
- DOI: 10.1001/jama.2023.24945
Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial
Abstract
Importance: The effect of continued treatment with tirzepatide on maintaining initial weight reduction is unknown.
Objective: To assess the effect of tirzepatide, with diet and physical activity, on the maintenance of weight reduction.
Design, setting, and participants: This phase 3, randomized withdrawal clinical trial conducted at 70 sites in 4 countries with a 36-week, open-label tirzepatide lead-in period followed by a 52-week, double-blind, placebo-controlled period included adults with a body mass index greater than or equal to 30 or greater than or equal to 27 and a weight-related complication, excluding diabetes.
Interventions: Participants (n = 783) enrolled in an open-label lead-in period received once-weekly subcutaneous maximum tolerated dose (10 or 15 mg) of tirzepatide for 36 weeks. At week 36, a total of 670 participants were randomized (1:1) to continue receiving tirzepatide (n = 335) or switch to placebo (n = 335) for 52 weeks.
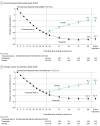
Main outcomes and measures: The primary end point was the mean percent change in weight from week 36 (randomization) to week 88. Key secondary end points included the proportion of participants at week 88 who maintained at least 80% of the weight loss during the lead-in period.
Results: Participants (n = 670; mean age, 48 years; 473 [71%] women; mean weight, 107.3 kg) who completed the 36-week lead-in period experienced a mean weight reduction of 20.9%. The mean percent weight change from week 36 to week 88 was -5.5% with tirzepatide vs 14.0% with placebo (difference, -19.4% [95% CI, -21.2% to -17.7%]; P < .001). Overall, 300 participants (89.5%) receiving tirzepatide at 88 weeks maintained at least 80% of the weight loss during the lead-in period compared with 16.6% receiving placebo (P < .001). The overall mean weight reduction from week 0 to 88 was 25.3% for tirzepatide and 9.9% for placebo. The most common adverse events were mostly mild to moderate gastrointestinal events, which occurred more commonly with tirzepatide vs placebo.
Conclusions and relevance: In participants with obesity or overweight, withdrawing tirzepatide led to substantial regain of lost weight, whereas continued treatment maintained and augmented initial weight reduction.
Trial registration: ClinicalTrials.gov Identifier: NCT04660643.
Conflict of interest statement
Figures
Comment in
-
Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity.JAMA. 2024 May 21;331(19):1673-1676. doi: 10.1001/jama.2024.2600. JAMA. 2024. PMID: 38630501 No abstract available.
-
Kann man die „Abnehm-Spritze“ absetzen?MMW Fortschr Med. 2024 Oct;166(17):24-25. doi: 10.1007/s15006-024-4362-9. MMW Fortschr Med. 2024. PMID: 39390167 Review. German. No abstract available.
References
-
- Garvey WT, Mechanick JI, Brett EM, et al. ; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1-203. doi: 10.4158/EP161365.GL - DOI - PubMed
-
- Rubino D, Abrahamsson N, Davies M, et al. ; STEP 4 Investigators . Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-1425. doi: 10.1001/jama.2021.3224 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

